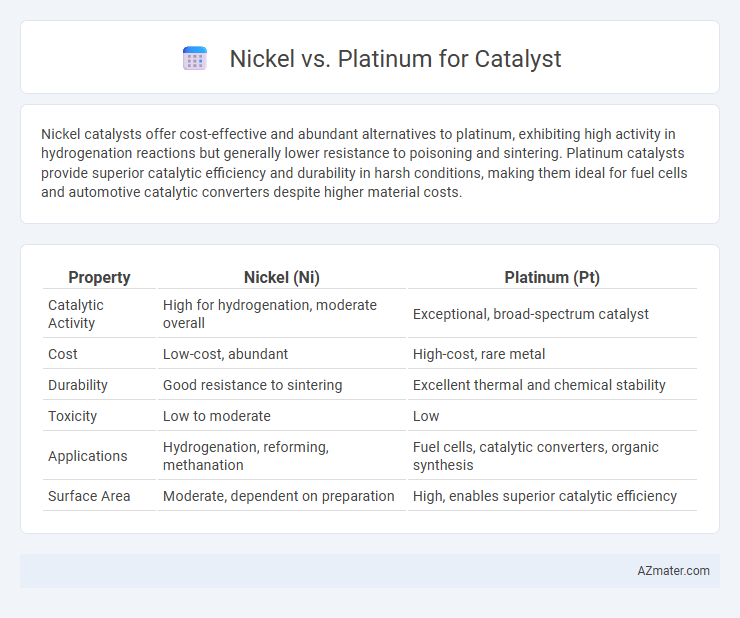
Nickel catalysts offer cost-effective and abundant alternatives to platinum, exhibiting high activity in hydrogenation reactions but generally lower resistance to poisoning and sintering. Platinum catalysts provide superior catalytic efficiency and durability in harsh conditions, making them ideal for fuel cells and automotive catalytic converters despite higher material costs.
Table of Comparison
| Property | Nickel (Ni) | Platinum (Pt) |
|---|---|---|
| Catalytic Activity | High for hydrogenation, moderate overall | Exceptional, broad-spectrum catalyst |
| Cost | Low-cost, abundant | High-cost, rare metal |
| Durability | Good resistance to sintering | Excellent thermal and chemical stability |
| Toxicity | Low to moderate | Low |
| Applications | Hydrogenation, reforming, methanation | Fuel cells, catalytic converters, organic synthesis |
| Surface Area | Moderate, dependent on preparation | High, enables superior catalytic efficiency |
Introduction to Catalysts: Nickel vs Platinum
Nickel and platinum are widely used catalysts in various industrial processes, with platinum offering superior catalytic efficiency and resistance to poisoning but at a significantly higher cost. Nickel serves as a cost-effective alternative, particularly in hydrogenation and reforming reactions, due to its adequate catalytic activity and abundance. The choice between nickel and platinum catalysts depends largely on the specific reaction conditions, desired selectivity, and economic considerations.
Chemical Properties and Reactivity
Nickel exhibits strong catalytic activity due to its ability to adsorb hydrogen and facilitate hydrogenation reactions, making it effective in processes like methanation and hydrogenolysis. Platinum, however, surpasses nickel in catalytic efficiency and selectivity due to its superior resistance to poisoning and ability to activate molecular oxygen, enhancing oxidation and reforming reactions. Chemically, nickel's high affinity for carbon can lead to catalyst deactivation through coking, whereas platinum's inertness and electron-rich surface sites contribute to its stability and durability in harsh reaction environments.
Catalyst Efficiency: Nickel Compared to Platinum
Nickel catalysts demonstrate lower activity and selectivity compared to platinum in hydrogenation reactions, resulting in reduced catalyst efficiency. Platinum catalysts offer superior resistance to poisoning and maintain higher stability under harsh reaction conditions, leading to prolonged catalytic performance. Although nickel is more cost-effective, platinum's enhanced catalytic efficiency makes it preferable for high-precision chemical processes.
Cost Analysis: Nickel vs Platinum Catalysts
Nickel catalysts offer a significantly lower cost compared to platinum catalysts, with nickel prices averaging around $20 per kilogram versus platinum's price exceeding $30,000 per kilogram. This dramatic price difference makes nickel an economically attractive option for large-scale industrial applications despite its generally lower catalytic activity and durability. Cost efficiency, combined with advancements in nickel catalyst performance, increasingly positions nickel as a competitive alternative in catalytic processes where budget constraints are critical.
Industrial Applications and Use Cases
Nickel catalysts are widely used in hydrogenation reactions, refinery processes, and methanation due to their cost-effectiveness and high activity, making them ideal for large-scale industrial applications. Platinum catalysts, known for superior stability and selectivity, are preferred in automotive catalytic converters, fuel cells, and fine chemical synthesis where precision and durability are critical. Industrial sectors leverage nickel for bulk petrochemical processing and platinum for high-value, precision catalytic functions in energy conversion and pollution control.
Environmental Impact and Sustainability
Nickel-based catalysts offer a more abundant and cost-effective option compared to platinum, reducing environmental strain related to mining and resource depletion. Platinum catalysts, while highly efficient and durable, carry a higher ecological footprint due to rare earth extraction and energy-intensive refining processes. Employing nickel catalysts contributes to greater sustainability by minimizing reliance on scarce metals and enabling more environmentally conscious manufacturing practices.
Durability and Longevity in Catalytic Processes
Platinum exhibits superior durability and longevity in catalytic processes due to its resistance to sintering and poisoning, maintaining high activity over extended cycles. Nickel, while more cost-effective, tends to degrade faster under harsh reaction conditions, experiencing metal agglomeration and carbon deposition that reduce catalyst lifespan. The enhanced stability of platinum catalysts supports prolonged industrial applications, making them preferable for sustained catalytic performance despite higher initial costs.
Regeneration and Recyclability
Nickel catalysts offer cost-effective regeneration through thermal treatment and can be recycled multiple times with minimal activity loss, making them suitable for large-scale industrial applications. Platinum catalysts demonstrate superior regeneration efficiency via oxidative and reductive treatments, retaining high catalytic activity after numerous cycles due to their enhanced resistance to sintering and poisoning. The recyclability of platinum catalysts is favored in high-value applications despite higher costs, while nickel provides a more economical solution with acceptable durability.
Recent Innovations in Catalyst Technology
Recent innovations in catalyst technology highlight nickel's growing prominence due to its cost-effectiveness and enhanced durability in hydrogenation reactions, driven by advances in nano-structuring and alloying techniques. Platinum catalysts, while traditionally favored for their superior activity and selectivity, face challenges with high costs and scarcity, prompting research into platinum-group metal alloys and core-shell structures to improve performance and reduce platinum content. Emerging studies also explore nickel-platinum hybrid catalysts that combine the strengths of both metals, achieving increased catalytic efficiency and stability for sustainable industrial applications.
Conclusion: Choosing the Right Catalyst
Nickel offers cost-effective catalytic performance with high activity for hydrogenation reactions, making it ideal for large-scale industrial processes where budget constraints are significant. Platinum provides superior catalytic efficiency, better resistance to poisoning, and longer lifespan, suited for precision applications requiring high selectivity and durability. Selecting the right catalyst depends on balancing economic factors with performance requirements, favoring nickel for cost-sensitive operations and platinum for high-value catalytic uses.
Infographic: Nickel vs Platinum for Catalyst
 azmater.com
azmater.com