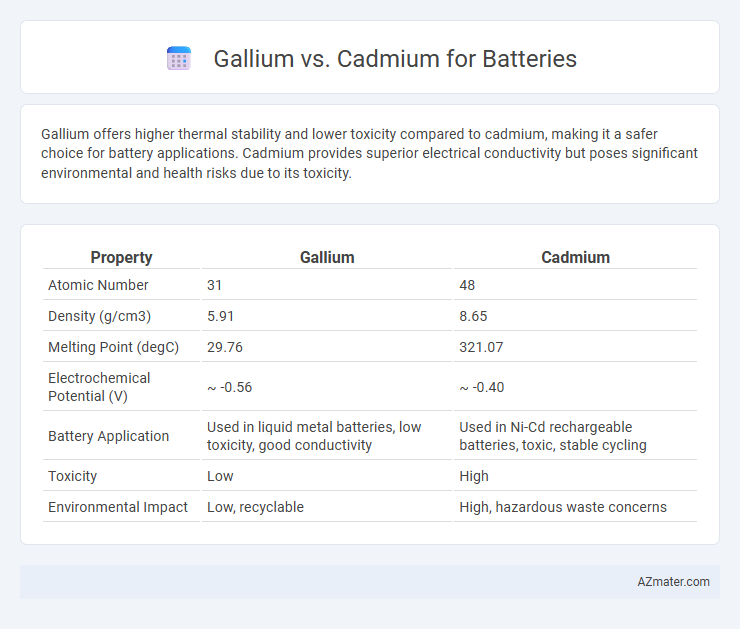
Gallium offers higher thermal stability and lower toxicity compared to cadmium, making it a safer choice for battery applications. Cadmium provides superior electrical conductivity but poses significant environmental and health risks due to its toxicity.
Table of Comparison
| Property | Gallium | Cadmium |
|---|---|---|
| Atomic Number | 31 | 48 |
| Density (g/cm3) | 5.91 | 8.65 |
| Melting Point (degC) | 29.76 | 321.07 |
| Electrochemical Potential (V) | ~ -0.56 | ~ -0.40 |
| Battery Application | Used in liquid metal batteries, low toxicity, good conductivity | Used in Ni-Cd rechargeable batteries, toxic, stable cycling |
| Toxicity | Low | High |
| Environmental Impact | Low, recyclable | High, hazardous waste concerns |
Introduction to Gallium and Cadmium in Battery Technology
Gallium and cadmium are emerging materials in advanced battery technology, influencing energy density and safety profiles. Gallium, a post-transition metal, offers high conductivity and low toxicity, making it suitable for liquid metal batteries and improving electrode stability. Cadmium, known for its use in nickel-cadmium (NiCd) batteries, provides reliable charge retention but raises environmental and health concerns due to its toxicity.
Chemical Properties: Gallium vs Cadmium
Gallium exhibits a low melting point of about 29.76degC and remains stable in air, with a strong affinity for forming alloys, which enhances battery electrode flexibility. Cadmium has a higher melting point of 321.07degC and forms stable compounds with high toxicity, influencing environmental risks in battery use. The electrochemical potentials of gallium (-0.55V) and cadmium (-0.40V) impact their respective battery voltage outputs and chemical stability during charge-discharge cycles.
Energy Density Comparison
Gallium-based batteries typically offer lower energy density compared to cadmium-based batteries, with cadmium cells such as nickel-cadmium (NiCd) batteries averaging around 45-80 Wh/kg, whereas gallium batteries remain under development with less established metrics. Cadmium's higher volumetric and gravimetric energy densities make NiCd batteries a more mature choice for applications requiring reliable power storage. Recent research into gallium's electrochemical properties aims to improve energy density, but Cd-based batteries currently dominate in established energy storage efficiency.
Environmental Impact and Toxicity
Gallium exhibits lower environmental toxicity compared to cadmium, making it a safer material for battery applications. Cadmium is highly toxic and poses significant environmental hazards, including soil and water contamination, which complicates disposal and recycling processes. Batteries incorporating gallium offer improved sustainability due to reduced risk of leaching harmful heavy metals into ecosystems.
Cycle Life and Durability
Gallium-enhanced batteries exhibit improved cycle life and durability due to gallium's ability to stabilize electrode materials and reduce dendrite formation, extending battery lifespan. Cadmium-based batteries, such as nickel-cadmium (NiCd) cells, offer robust cycle performance but suffer from memory effect and environmental toxicity that limit long-term durability. Advances in gallium-doped battery technology demonstrate higher retention capacity and resistance to structural degradation compared to traditional cadmium batteries, positioning gallium as a promising element for next-generation energy storage solutions.
Cost Analysis: Gallium vs Cadmium Batteries
Gallium batteries typically incur higher initial costs due to the scarcity and complex extraction processes of gallium compared to cadmium, which is more abundant and established in battery technologies like nickel-cadmium (NiCd). However, gallium-based batteries offer longer lifespan and better thermal stability, potentially reducing long-term expenses despite higher upfront investment. Cadmium batteries are more cost-efficient for short-term applications but face environmental disposal costs due to cadmium's toxicity, impacting overall cost-effectiveness.
Safety Considerations in Battery Applications
Gallium offers superior safety in battery applications due to its non-toxic nature and low environmental impact compared to Cadmium, which is highly toxic and poses significant health and ecological risks. Cadmium-based batteries require stringent handling and recycling protocols to mitigate contamination and hazardous exposure, whereas Gallium-based batteries typically exhibit enhanced thermal stability and reduced risk of leakage or combustion. Safety considerations prioritize Gallium for innovative, eco-friendly battery technologies, while Cadmium remains heavily regulated owing to its toxicity and environmental hazards.
Scalability and Material Availability
Gallium offers limited scalability for battery applications due to its relatively low natural abundance and high cost, restricting large-scale production. Cadmium, while more abundant and cheaper, faces significant environmental and health concerns that limit its widespread use despite better material availability. Sustainable battery development favors materials with abundant supply chains and lower toxicity, where gallium's scarcity and cadmium's environmental impact pose notable challenges.
Current Market Applications and Trends
Gallium is increasingly explored in advanced battery technologies for its ability to form stable alloys with lithium, improving battery lifespan and energy density. Cadmium remains a critical component in nickel-cadmium (NiCd) rechargeable batteries, widely used in portable electronics and power tools due to its reliability and cost efficiency. Market trends show a gradual shift towards gallium-enhanced batteries in emerging sectors like electric vehicles and grid storage, driven by the demand for higher performance and environmental compliance.
Future Prospects: Gallium and Cadmium in Advanced Batteries
Gallium and cadmium both hold promising potential for future battery technologies due to their unique electrochemical properties. Gallium offers advantages in liquid metal batteries with high energy density and recyclability, while cadmium remains relevant in nickel-cadmium (NiCd) batteries for reliable performance and durability. Advances in materials science aim to optimize gallium's conductivity and cadmium's toxicity mitigation, driving next-generation battery innovation for grid storage and portable electronics.
Infographic: Gallium vs Cadmium for Battery
 azmater.com
azmater.com