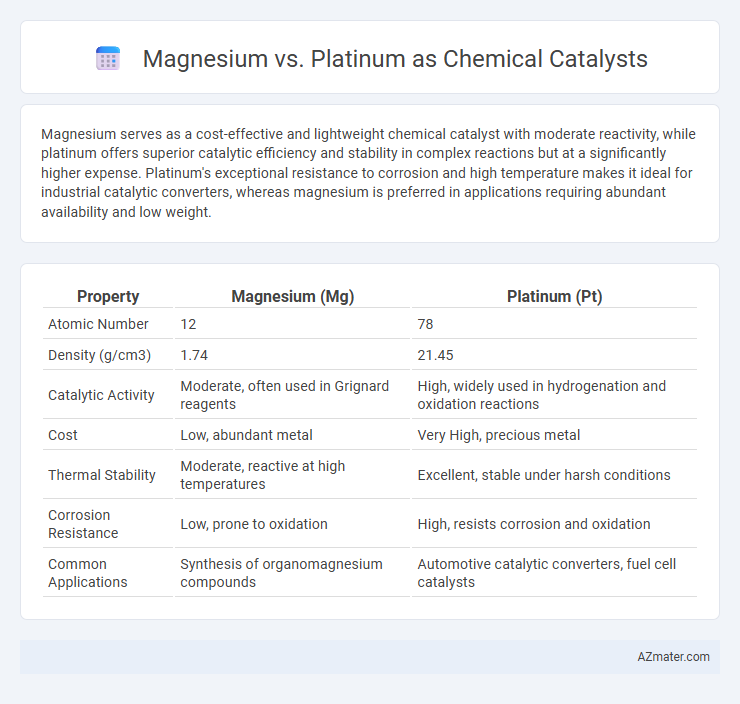
Magnesium serves as a cost-effective and lightweight chemical catalyst with moderate reactivity, while platinum offers superior catalytic efficiency and stability in complex reactions but at a significantly higher expense. Platinum's exceptional resistance to corrosion and high temperature makes it ideal for industrial catalytic converters, whereas magnesium is preferred in applications requiring abundant availability and low weight.
Table of Comparison
| Property | Magnesium (Mg) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 12 | 78 |
| Density (g/cm3) | 1.74 | 21.45 |
| Catalytic Activity | Moderate, often used in Grignard reagents | High, widely used in hydrogenation and oxidation reactions |
| Cost | Low, abundant metal | Very High, precious metal |
| Thermal Stability | Moderate, reactive at high temperatures | Excellent, stable under harsh conditions |
| Corrosion Resistance | Low, prone to oxidation | High, resists corrosion and oxidation |
| Common Applications | Synthesis of organomagnesium compounds | Automotive catalytic converters, fuel cell catalysts |
Introduction to Magnesium and Platinum as Chemical Catalysts
Magnesium serves as an effective chemical catalyst in organic synthesis by facilitating Grignard reactions due to its ability to form organomagnesium compounds that readily react with carbonyl groups. Platinum, a noble metal catalyst, excels in hydrogenation processes and catalytic converters because of its high catalytic activity and resistance to poisoning under various reaction conditions. Both metals offer unique reactivity profiles, with magnesium favoring nucleophilic addition and platinum promoting surface-mediated redox reactions.
Atomic Structure and Catalytic Properties
Magnesium, with its atomic number 12 and electron configuration [Ne] 3s2, exhibits lower d-orbital availability, resulting in limited catalytic activity compared to platinum, which has an atomic number 78 and electron configuration [Xe] 4f14 5d9 6s1, providing a rich d-electron environment that facilitates diverse adsorption and activation of reactant molecules. Platinum's densely packed face-centered cubic (FCC) lattice and high surface atom density enhance its ability to act as an effective catalyst in hydrogenation and oxidation reactions, while magnesium's more reactive s-block character primarily influences its use as a sacrificial agent rather than as a conventional catalyst. Catalytic properties of platinum, such as high stability and resistance to poisoning, outperform magnesium, which shows limited catalytic versatility due to its larger atomic radius and lower electronegativity.
Abundance and Economic Considerations
Magnesium is significantly more abundant in the Earth's crust, constituting about 2.1% by weight, while platinum is extremely rare, with an abundance of roughly 5 parts per billion. The lower cost and greater availability of magnesium make it economically favorable for large-scale catalytic applications, whereas platinum's scarcity and high price limit its use to specialized, high-value processes. Economic considerations often prioritize magnesium for sustainable and cost-effective catalyst production despite platinum's superior catalytic performance in certain reactions.
Catalytic Activity: Magnesium vs Platinum
Platinum exhibits significantly higher catalytic activity than magnesium due to its superior ability to adsorb and activate reactant molecules, facilitating faster reaction rates in processes such as hydrogenation and oxidation. Magnesium, while less active, serves as a catalyst in specific reactions by providing basic sites that promote deprotonation and other surface interactions, but its overall turnover frequency is lower compared to platinum. Platinum's d-orbitals enable multiple bonding interactions with intermediates, increasing catalytic efficiency, whereas magnesium's s-block electronic structure limits its catalytic versatility.
Selectivity and Reaction Pathways
Magnesium catalysts exhibit high selectivity for hydrogenation reactions by favoring pathways with lower activation energies, often yielding fewer side products compared to platinum. Platinum catalysts enable diverse reaction pathways due to their versatile surface chemistry, but this versatility can reduce selectivity by promoting multiple competing reactions. In selective catalysis, magnesium's ability to direct reaction mechanisms selectively contrasts with platinum's broader activity profile, impacting product distribution and efficiency.
Stability and Durability in Catalytic Processes
Magnesium-based catalysts exhibit moderate stability but can degrade under high-temperature or oxidative conditions, limiting their durability in prolonged catalytic processes. Platinum catalysts demonstrate superior thermal and chemical stability, maintaining high durability and consistent performance under harsh reaction environments. This enhanced stability of platinum makes it a preferred choice for industrial catalytic applications requiring long-term reliability.
Environmental Impact of Catalyst Usage
Magnesium catalysts are favored for their low environmental impact due to their abundance, biodegradability, and low toxicity, resulting in less harmful waste and reduced ecological footprint. Platinum catalysts, although highly efficient and durable, pose significant environmental concerns because of their scarcity, high energy-intensive extraction processes, and challenges in recycling, which contribute to resource depletion and increased carbon emissions. The choice between magnesium and platinum catalysts heavily influences the sustainability of chemical processes, with magnesium offering a greener alternative for eco-friendly catalysis.
Industrial Applications: Magnesium vs Platinum
Platinum serves as a highly efficient chemical catalyst in industrial applications such as catalytic converters, petroleum refining, and the production of nitric acid due to its excellent resistance to corrosion and ability to accelerate reactions at lower temperatures. Magnesium, while less expensive, is primarily utilized in specialty chemical syntheses and as a reagent in organometallic catalysis, but it lacks the durability and broad catalytic versatility of platinum. The higher catalytic activity, stability under harsh industrial conditions, and recyclability of platinum make it the preferred choice despite its greater cost.
Advances in Catalyst Design and Engineering
Recent advances in catalyst design highlight the superior selectivity of platinum over magnesium for catalytic reactions involving hydrogenation and oxidation. Nanostructured platinum catalysts demonstrate enhanced active site exposure and stability, driving improved reaction rates and product yields. Engineering efforts have focused on alloying platinum with other metals to optimize electronic properties, whereas magnesium catalysts remain limited by lower activity and thermal stability.
Future Trends and Research Directions
Emerging research in chemical catalysis highlights magnesium's potential as a sustainable and cost-effective alternative to platinum, driven by its abundance and lower environmental impact. Future trends focus on enhancing magnesium-based catalysts' activity and selectivity through nanostructuring and alloying techniques to rival platinum's superior performance. Advances in computational catalysis and in situ characterization are accelerating the development of hybrid catalytic systems that integrate magnesium with trace amounts of platinum to optimize efficiency and durability.
Infographic: Magnesium vs Platinum for Chemical Catalyst
 azmater.com
azmater.com