Vanadium electrodes offer superior energy density and longer cycle life compared to nickel, making them ideal for high-performance batteries. Nickel provides high conductivity and cost-efficiency but suffers from faster capacity degradation in battery applications.
Table of Comparison
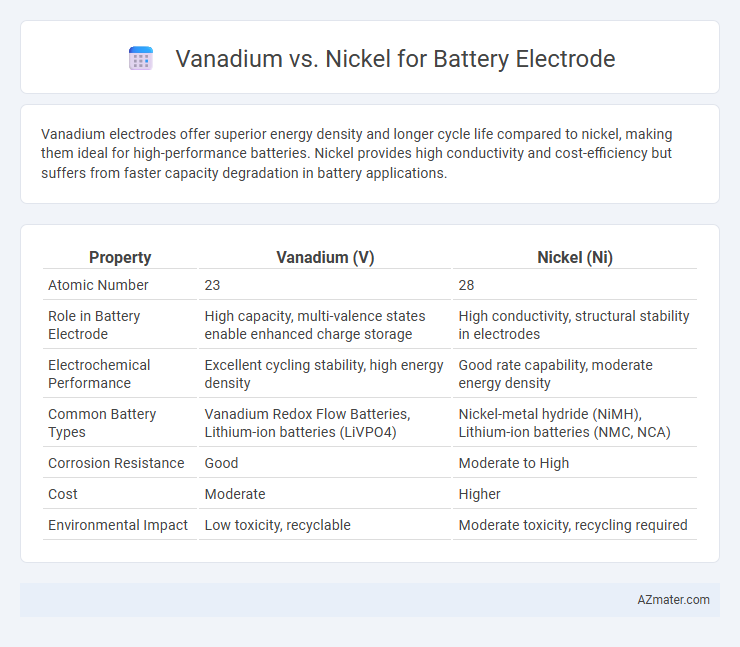
| Property | Vanadium (V) | Nickel (Ni) |
|---|---|---|
| Atomic Number | 23 | 28 |
| Role in Battery Electrode | High capacity, multi-valence states enable enhanced charge storage | High conductivity, structural stability in electrodes |
| Electrochemical Performance | Excellent cycling stability, high energy density | Good rate capability, moderate energy density |
| Common Battery Types | Vanadium Redox Flow Batteries, Lithium-ion batteries (LiVPO4) | Nickel-metal hydride (NiMH), Lithium-ion batteries (NMC, NCA) |
| Corrosion Resistance | Good | Moderate to High |
| Cost | Moderate | Higher |
| Environmental Impact | Low toxicity, recyclable | Moderate toxicity, recycling required |
Introduction to Battery Electrode Materials
Vanadium and nickel are both critical materials for battery electrodes, each offering distinct electrochemical properties that impact battery performance. Vanadium is prized for its high redox stability and multiple oxidation states, making it ideal for vanadium redox flow batteries with long cycle life and high energy efficiency. Nickel provides excellent electrical conductivity and energy density, commonly employed in nickel-metal hydride (NiMH) and lithium nickel manganese cobalt oxide (NMC) cathodes, enhancing overall battery capacity and power output.
Overview of Vanadium in Battery Technology
Vanadium is increasingly recognized for its exceptional role in redox flow batteries, offering high energy density and long cycle life essential for grid-scale energy storage. Compared to nickel, vanadium's stable valence states minimize capacity degradation and enable efficient charge-discharge cycles in vanadium redox flow batteries (VRFBs). Its abundant availability and scalability make vanadium a cost-effective and sustainable choice for sustainable battery electrode applications.
Role of Nickel in Battery Electrodes
Nickel plays a critical role in battery electrodes by enhancing energy density and cycling stability, particularly in lithium-ion and nickel-metal hydride batteries. Its high electrochemical activity and ability to undergo reversible redox reactions improve electrode capacity and charge retention. Compared to vanadium, nickel offers superior conductivity and contributes to faster charge-discharge rates, making it essential for high-performance energy storage systems.
Electrochemical Properties: Vanadium vs Nickel
Vanadium exhibits superior electrochemical properties compared to nickel due to its multiple stable oxidation states, allowing enhanced redox flexibility and higher capacity retention in battery electrodes. Nickel, while offering good electrical conductivity and moderate capacity, generally suffers from lower cycling stability and reduced electrochemical efficiency under high current densities. Vanadium-based electrodes provide improved energy density and faster ion diffusion kinetics, making them more suitable for advanced rechargeable battery applications.
Energy Density Comparison
Vanadium and nickel are critical materials for battery electrodes, yet they differ notably in energy density. Nickel-based electrodes typically offer higher volumetric and gravimetric energy densities, often exceeding 200 Wh/kg, making them suitable for applications demanding compact and lightweight energy storage. Vanadium, primarily used in vanadium redox flow batteries, provides lower energy densities around 25-50 Wh/kg but excels in scalability and long cycle life for large-scale energy storage systems.
Cycle Life and Stability Analysis
Vanadium-based electrodes exhibit superior cycle life and electrochemical stability compared to nickel counterparts due to their robust crystal structure and higher resistance to capacity fading. Vanadium redox flow batteries demonstrate prolonged charge-discharge cycles exceeding 10,000 with minimal performance degradation, whereas nickel-based electrodes often suffer from faster capacity decay within 1,000 to 2,000 cycles. Stability analysis reveals vanadium's enhanced tolerance to oxidative and thermal stress, making it a preferred material for long-lasting battery electrode applications.
Cost and Material Availability
Vanadium offers a cost-effective advantage over nickel due to its abundant global reserves and lower market price, making it a more sustainable choice for large-scale battery electrode production. Nickel, while popular for its high energy density contribution, faces price volatility and supply constraints linked to geopolitical factors and concentrated mining regions. Material availability for vanadium ensures more stable supply chains, reducing risks in electrode manufacturing compared to nickel's dependence on specific extraction sites.
Environmental Impact and Sustainability
Vanadium batteries offer superior environmental sustainability due to their longer cycle life and easier recyclability compared to nickel-based electrodes, which rely heavily on mining practices that contribute to significant ecological degradation and carbon emissions. The extraction of nickel often involves harmful processes such as acid drainage and habitat destruction, intensifying the environmental footprint of nickel-based battery production. Vanadium's abundance and less energy-intensive extraction process make it a more eco-friendly choice for battery electrodes, promoting sustainable energy storage solutions.
Future Trends in Electrode Material Development
Vanadium and nickel are critical metals in the evolution of battery electrode materials, with vanadium gaining attention for its high energy density and cycle stability in vanadium redox flow batteries, ideal for large-scale energy storage. Nickel remains a dominant choice in lithium-ion batteries due to its high specific capacity and cost-effectiveness, but concerns about resource scarcity and environmental impact drive research towards optimizing nickel utilization and finding sustainable alternatives. Future trends focus on hybrid metal electrodes combining vanadium and nickel to leverage their complementary electrochemical properties, alongside advances in nanostructuring and doping techniques to enhance electrode performance and lifespan.
Conclusion: Choosing Vanadium or Nickel
Vanadium offers superior energy density and enhanced cycle stability, making it ideal for long-lasting, high-performance battery electrodes. Nickel provides cost-effective conductivity and faster charge rates, suited for applications prioritizing affordability and quick energy delivery. Selecting between vanadium and nickel depends on balancing performance needs with budget constraints and application-specific energy requirements.
Infographic: Vanadium vs Nickel for Battery Electrode
 azmater.com
azmater.com