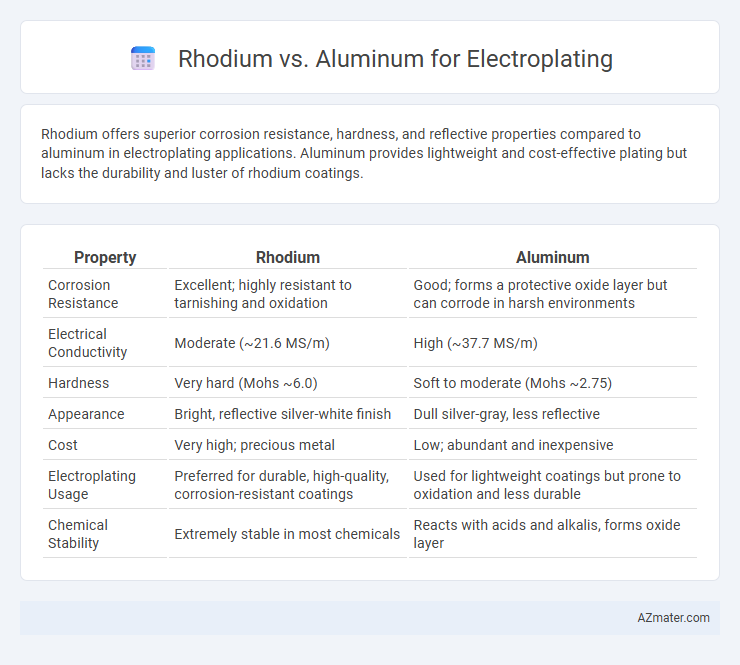
Rhodium offers superior corrosion resistance, hardness, and reflective properties compared to aluminum in electroplating applications. Aluminum provides lightweight and cost-effective plating but lacks the durability and luster of rhodium coatings.
Table of Comparison
| Property | Rhodium | Aluminum |
|---|---|---|
| Corrosion Resistance | Excellent; highly resistant to tarnishing and oxidation | Good; forms a protective oxide layer but can corrode in harsh environments |
| Electrical Conductivity | Moderate (~21.6 MS/m) | High (~37.7 MS/m) |
| Hardness | Very hard (Mohs ~6.0) | Soft to moderate (Mohs ~2.75) |
| Appearance | Bright, reflective silver-white finish | Dull silver-gray, less reflective |
| Cost | Very high; precious metal | Low; abundant and inexpensive |
| Electroplating Usage | Preferred for durable, high-quality, corrosion-resistant coatings | Used for lightweight coatings but prone to oxidation and less durable |
| Chemical Stability | Extremely stable in most chemicals | Reacts with acids and alkalis, forms oxide layer |
Introduction to Electroplating
Electroplating involves depositing a thin layer of metal onto a substrate using an electric current to improve corrosion resistance, enhance appearance, and increase surface hardness. Rhodium electroplating offers superior reflectivity, exceptional hardness, and excellent resistance to tarnishing compared to aluminum, which is less commonly used due to its lower conductivity and difficulty in plating processes. The choice between rhodium and aluminum plating depends on the desired durability, finish quality, and application requirements in industries such as jewelry, automotive, and electronics.
Overview of Rhodium as a Plating Material
Rhodium, a rare and precious metal in the platinum group, is prized for its exceptional hardness, corrosion resistance, and brilliant reflective finish, making it ideal for electroplating high-end jewelry and automotive parts. Its ability to create a durable, tarnish-resistant coating significantly enhances the longevity and aesthetic appeal of plated items. Despite higher costs, rhodium plating offers superior protection against oxidation compared to aluminum, ensuring long-lasting performance in demanding applications.
Overview of Aluminum as a Plating Material
Aluminum serves as a popular base material in electroplating due to its lightweight properties, excellent corrosion resistance, and high conductivity. Its surface forms a natural oxide layer that requires specific pre-treatment processes like etching or zincating to ensure strong adhesion of plating metals. Although aluminum can be plated with various metals, achieving uniform coverage and long-lasting durability demands advanced techniques tailored to its reactive surface characteristics.
Electroplating Process: Rhodium vs Aluminum
Rhodium electroplating involves depositing a thin, corrosion-resistant, and highly reflective layer using an aqueous rhodium salt solution with a controlled current and temperature, ensuring uniform coverage and enhanced durability. Aluminum electroplating is less common due to its high reactivity and difficulty forming consistent deposits; it typically requires specialized non-aqueous electrolyte baths and precise process parameters to achieve effective plating. Rhodium plating offers superior hardness and oxidation resistance compared to aluminum, making it the preferred choice in jewelry and electronics applications.
Durability and Corrosion Resistance Comparison
Rhodium offers superior durability and corrosion resistance compared to aluminum when used in electroplating, providing a hard, dense, and chemically inert surface that effectively resists tarnishing and wear. Aluminum coatings, while lightweight and a good conductor, are more prone to oxidation and corrosion over time, especially in harsh environments or exposure to moisture. The exceptional hardness and non-reactive properties of rhodium make it ideal for applications demanding long-lasting, protective finishes.
Electrical Conductivity Differences
Rhodium exhibits significantly higher electrical conductivity compared to aluminum, making it a superior choice for electroplating in applications requiring efficient current flow and minimal resistance. While aluminum offers good conductivity at a lower cost, its oxide layer can hinder consistent electrical performance in electroplating processes. Rhodium's exceptional corrosion resistance and stable conductive properties ensure enhanced durability and optimal electrical contact in high-precision, high-performance components.
Cost Analysis: Rhodium vs Aluminum Plating
Rhodium plating commands a significantly higher price compared to aluminum due to its rarity and superior corrosion resistance, with market costs often exceeding $10,000 per ounce versus aluminum's $1.50 per pound. While rhodium provides exceptional durability and a brilliant, reflective finish ideal for high-end jewelry and automotive parts, aluminum plating offers a cost-effective solution with moderate corrosion protection suited for industrial applications. Selecting between rhodium and aluminum plating hinges on balancing budget constraints against the performance demands of the finished product.
Common Applications in Industry
Rhodium electroplating is commonly used in the automotive and jewelry industries for its exceptional corrosion resistance, high reflectivity, and superior hardness, providing a durable, mirror-like finish on engine components and fine jewelry pieces. Aluminum electroplating finds its primary applications in aerospace and electronics, where lightweight, conductive coatings enhance corrosion protection and electrical performance on aircraft parts and electronic connectors. Both metals serve distinct roles in electroplating, with rhodium favored for its aesthetic and wear-resistant properties, while aluminum is chosen for functional, lightweight, and conductive surface treatments.
Environmental Impact and Safety Considerations
Rhodium electroplating generates less hazardous waste compared to aluminum, as rhodium baths typically use fewer toxic chemicals and produce lower heavy metal contamination. Aluminum plating requires highly alkaline solutions and aggressive etchants, increasing the risk of chemical burns and environmental pollution during disposal. Safety considerations favor rhodium plating due to its stable, less corrosive electrolytes and reduced emission of toxic fumes, making it a more environmentally friendly and safer option for industrial applications.
Choosing the Right Metal for Your Electroplating Needs
Rhodium offers exceptional corrosion resistance and a brilliant, reflective finish, making it ideal for high-end jewelry and electronics requiring durability and aesthetic appeal. Aluminum, while cost-effective and lightweight, is less corrosion-resistant and requires special surface preparation to ensure proper adhesion in electroplating. Selecting the right metal depends on the application's durability requirements, budget constraints, and desired visual qualities.
Infographic: Rhodium vs Aluminum for Electroplating
 azmater.com
azmater.com