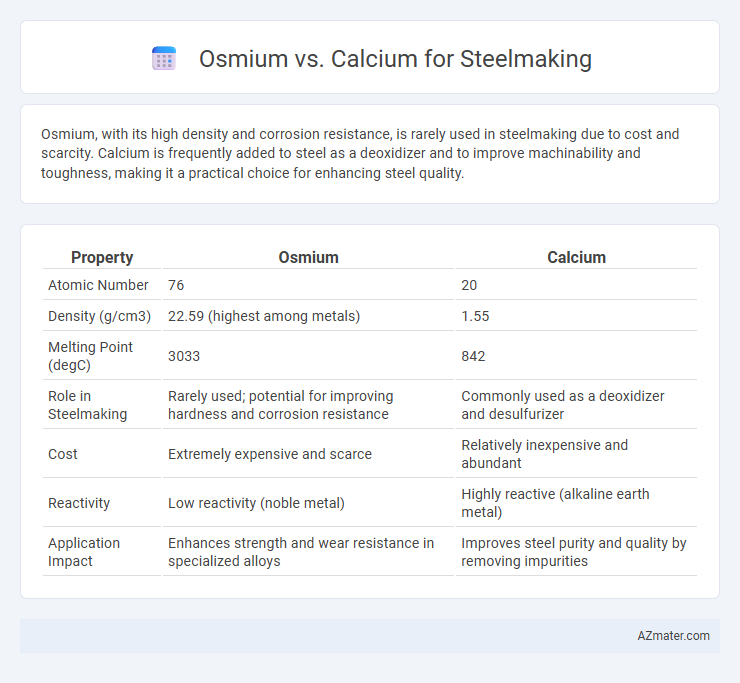
Osmium, with its high density and corrosion resistance, is rarely used in steelmaking due to cost and scarcity. Calcium is frequently added to steel as a deoxidizer and to improve machinability and toughness, making it a practical choice for enhancing steel quality.
Table of Comparison
| Property | Osmium | Calcium |
|---|---|---|
| Atomic Number | 76 | 20 |
| Density (g/cm3) | 22.59 (highest among metals) | 1.55 |
| Melting Point (degC) | 3033 | 842 |
| Role in Steelmaking | Rarely used; potential for improving hardness and corrosion resistance | Commonly used as a deoxidizer and desulfurizer |
| Cost | Extremely expensive and scarce | Relatively inexpensive and abundant |
| Reactivity | Low reactivity (noble metal) | Highly reactive (alkaline earth metal) |
| Application Impact | Enhances strength and wear resistance in specialized alloys | Improves steel purity and quality by removing impurities |
Introduction to Osmium and Calcium in Steelmaking
Osmium, a dense platinum-group metal, offers exceptional hardness and corrosion resistance when alloyed with steel, enhancing durability in specialized applications. Calcium serves as a powerful deoxidizer and desulfurizing agent in steelmaking, improving castability and cleanliness by removing impurities and modifying inclusions. Both elements play distinct roles, with osmium enhancing mechanical properties and calcium optimizing metallurgical quality in steel production.
Chemical Properties Relevant to Steel Production
Osmium exhibits exceptional hardness and high melting point, making it resistant to oxidation and corrosion, but its scarcity and cost limit its use in steelmaking. Calcium, with its strong deoxidizing and desulfurizing properties, enhances steel quality by removing impurities and improving ductility. The chemical affinity of calcium for oxygen and sulfur makes it a crucial additive in modern steel refinement processes, unlike osmium which is rarely applied due to economic and practical constraints.
Abundance and Availability in Nature
Osmium is an extremely rare and dense metal with an abundance of approximately 0.001 parts per million in the Earth's crust, making it scarce and expensive for steelmaking applications. Calcium, by contrast, is far more abundant, with about 41,500 parts per million, commonly found in minerals like limestone and gypsum, making it readily available and cost-effective for industrial uses. The significant disparity in natural abundance and availability strongly favors calcium as a practical alloying element in steel production over osmium.
Extraction and Cost Comparison
Osmium, a rare and dense platinum-group metal, is not commonly used in steelmaking due to its high extraction costs and scarcity, whereas calcium is widely utilized as a deoxidizer and desulfurizer because of its abundance and low price. Extracting osmium involves complex processes like fractional distillation of crude platinum ore, leading to significant expenses, while calcium is produced on an industrial scale through electrolysis of molten calcium chloride, making it more cost-effective for large-scale steel production. The cost difference is substantial, with osmium priced at thousands of dollars per ounce compared to calcium's relatively minimal production cost, reinforcing calcium's practicality in the steel industry.
Impact on Steel Microstructure
Osmium significantly enhances steel microstructure by refining grain size and increasing hardness due to its high melting point and density, leading to improved wear resistance and strength. Calcium acts as a powerful deoxidizer and inclusion modifier, promoting non-metallic inclusion coagulation that results in cleaner steel and improved toughness. The combined effects of osmium and calcium optimize steel's mechanical properties and corrosion resistance through refined grain boundaries and reduced impurity levels.
Influence on Mechanical Properties
Osmium, known for its exceptional density and hardness, enhances steel's wear resistance and tensile strength when used as an alloying element but is rarely used due to its rarity and high cost. Calcium, on the other hand, improves steel's machinability and toughness by modifying non-metallic inclusions, reducing brittleness and enhancing ductility. The choice between osmium and calcium in steelmaking significantly impacts mechanical properties such as hardness, toughness, and resistance to fracture.
Environmental Considerations and Safety
Osmium and calcium differ significantly in environmental impact and safety during steelmaking; osmium is rare, toxic, and poses substantial handling risks due to its volatile osmium tetroxide, while calcium is abundant, less hazardous, and widely used as a deoxidizer in steel production. The environmental footprint of osmium mining is considerably higher due to scarcity and energy-intensive extraction, whereas calcium production is more sustainable with lower emissions. Steelmakers prefer calcium for its safer, eco-friendly profile, minimizing toxic waste and ensuring compliance with environmental regulations.
Industrial Applications and Use Cases
Osmium, with its exceptional density and hardness, is rarely used in steelmaking due to extreme cost and scarcity, limiting its industrial applications to niche fields such as catalysis and high-precision alloys. Calcium plays a vital role in steel production as a deoxidizer and desulfurizing agent, improving steel quality by refining grain structure and enhancing mechanical properties. Industrial use of calcium in steelmaking is widespread, making it critical for producing cleaner, more ductile steel in large-scale manufacturing processes.
Limitations and Challenges of Each Element
Osmium's extreme rarity and high cost significantly limit its practical application in steelmaking despite its exceptional hardness and corrosion resistance. Calcium, while economically viable and effective as a deoxidizer and desulfurizer, poses challenges due to its high reactivity, which can cause unwanted inclusions and inconsistent alloy properties. The difficulty in controlling precise quantities of these elements further complicates their integration, impacting steel quality and production efficiency.
Future Prospects in Steel Alloying
Osmium's exceptional hardness and high melting point offer promising potential for enhancing steel alloys' wear resistance and thermal stability in future steelmaking applications. Calcium, known for its strong deoxidizing and desulfurizing properties, is vital in refining steel cleanliness and improving machinability, making it indispensable in modern alloy production. Advancements in nanotechnology and metallurgical processes could enable the controlled incorporation of osmium and calcium to develop high-performance steels with superior durability and corrosion resistance.
Infographic: Osmium vs Calcium for Steelmaking
 azmater.com
azmater.com