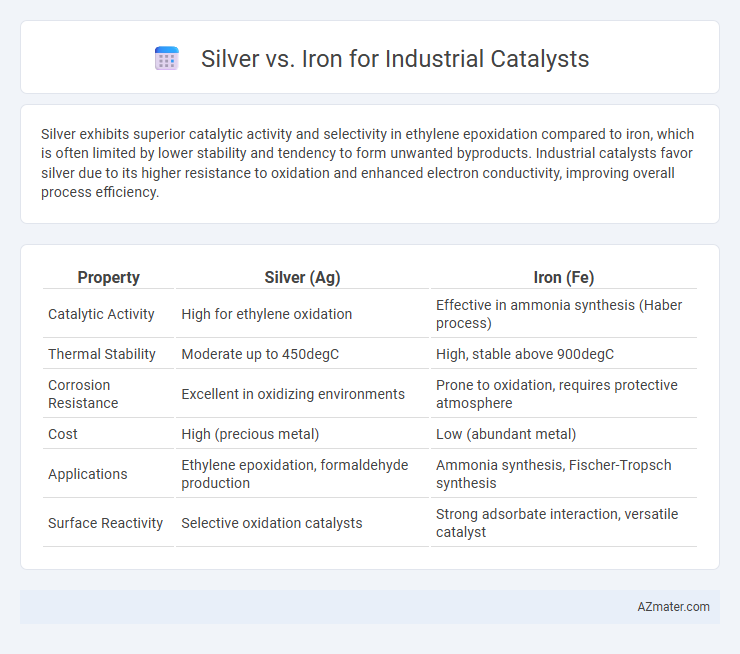
Silver exhibits superior catalytic activity and selectivity in ethylene epoxidation compared to iron, which is often limited by lower stability and tendency to form unwanted byproducts. Industrial catalysts favor silver due to its higher resistance to oxidation and enhanced electron conductivity, improving overall process efficiency.
Table of Comparison
| Property | Silver (Ag) | Iron (Fe) |
|---|---|---|
| Catalytic Activity | High for ethylene oxidation | Effective in ammonia synthesis (Haber process) |
| Thermal Stability | Moderate up to 450degC | High, stable above 900degC |
| Corrosion Resistance | Excellent in oxidizing environments | Prone to oxidation, requires protective atmosphere |
| Cost | High (precious metal) | Low (abundant metal) |
| Applications | Ethylene epoxidation, formaldehyde production | Ammonia synthesis, Fischer-Tropsch synthesis |
| Surface Reactivity | Selective oxidation catalysts | Strong adsorbate interaction, versatile catalyst |
Introduction to Industrial Catalysts
Silver and iron serve distinct roles in industrial catalysts due to their unique chemical properties and reactivity. Silver catalysts excel in selective oxidation processes, such as ethylene epoxidation, owing to their high oxygen activation ability and resistance to deactivation. Iron catalysts, widely used in Haber-Bosch ammonia synthesis and Fischer-Tropsch reactions, provide cost-effective catalytic activity with excellent redox flexibility essential for nitrogen fixation and hydrocarbon conversion.
Overview of Silver and Iron as Catalysts
Silver and iron are widely utilized as industrial catalysts due to their distinct catalytic properties and applications. Silver excels in selective oxidation reactions, particularly in ethylene epoxidation, owing to its high activity and selectivity. Iron is favored in processes like ammonia synthesis and Fischer-Tropsch catalysis because of its abundance, cost-effectiveness, and ability to facilitate various redox reactions efficiently.
Chemical Properties Relevant to Catalysis
Silver exhibits high electrical conductivity and moderate catalytic activity, particularly effective in partial oxidation and selective oxidation reactions due to its ability to adsorb oxygen while resisting over-oxidation. Iron, with its multiple oxidation states (Fe2+, Fe3+), provides versatile redox properties, facilitating electron transfer in processes like Fischer-Tropsch synthesis and ammonia synthesis. The distinct surface chemistry of silver promotes selective catalysis with minimal side reactions, whereas iron's robust oxophilicity enhances catalytic cycles involving oxygen activation and hydrogenation.
Common Industrial Applications
Silver catalysts are extensively utilized in ethylene oxide production due to their high selectivity and activity, making them ideal for the chemical industry. Iron catalysts are predominantly employed in the Haber-Bosch process for ammonia synthesis and Fischer-Tropsch synthesis for hydrocarbon production, essential for fertilizer and fuel manufacturing. Both metals, with silver favored for oxidation reactions and iron for nitrogen fixation and hydrocarbon conversion, play pivotal roles in industrial catalytic processes.
Catalytic Efficiency: Silver vs Iron
Silver exhibits higher catalytic efficiency than iron in oxidation reactions due to its superior oxygen activation and selectivity, especially in ethylene epoxidation processes. Iron catalysts, while abundant and cost-effective, often suffer from lower activity and selectivity, requiring harsher operating conditions to achieve comparable results. The unique electronic structure of silver enhances reaction rates and product yield, making it the preferred choice for high-performance industrial catalysis.
Cost and Availability Comparison
Silver catalysts typically offer higher activity for selective oxidation reactions but come with significantly higher costs due to silver's scarcity and market price volatility. Iron catalysts, in contrast, are more abundant and inexpensive, providing cost-effective options for large-scale industrial processes despite often lower catalytic efficiency. The availability of iron ensures sustainable supply chains, while silver's limited reserves can lead to supply constraints and increased operational expenses.
Environmental Impact and Sustainability
Silver catalysts exhibit lower toxicity and higher recyclability compared to iron catalysts, reducing environmental hazards during industrial processes. Iron, being abundant and inexpensive, offers a sustainable raw material source, but its catalytic efficiency often necessitates higher energy consumption and generates more waste. Optimizing silver catalysts can enhance green chemistry applications by minimizing harmful emissions and facilitating catalyst recovery, thereby supporting sustainable industrial development.
Durability and Longevity in Industrial Use
Silver catalysts exhibit superior durability in industrial applications due to their high resistance to oxidation and corrosion, extending operational lifespan significantly compared to iron catalysts. Iron catalysts often suffer from faster degradation under high-temperature and oxidative conditions, leading to more frequent replacements and increased maintenance costs. The enhanced longevity of silver catalysts improves process efficiency and reduces downtime in catalytic systems used for chemical synthesis and emission control.
Recent Advances and Innovations
Recent advances in industrial catalysts highlight silver's superior selectivity in ethylene epoxidation, driven by enhanced nanoscale structuring and surface modifications that improve oxygen activation and reduce carbon dioxide formation. Innovations in iron-based catalysts focus on cost-effective, earth-abundant alternatives for Fischer-Tropsch synthesis and ammonia production, leveraging dopants and optimized supports to enhance activity and stability under harsh conditions. Comparative studies reveal silver's dominance in oxidation reactions, while iron catalysts gain traction in sustainable processes, reflecting a strategic balance between performance and environmental impact in industrial applications.
Choosing the Right Catalyst: Key Considerations
Choosing the right catalyst for industrial applications depends on factors such as reaction temperature, selectivity, and cost-efficiency. Silver catalysts offer superior performance in ethylene epoxidation due to high selectivity and resistance to sintering, whereas iron catalysts excel in Fischer-Tropsch synthesis due to their ability to promote hydrocarbon chain growth and tolerance to water. Evaluating catalyst stability, activity, and feedstock compatibility is essential for optimizing process efficiency and product yield.
Infographic: Silver vs Iron for Industrial Catalyst
 azmater.com
azmater.com