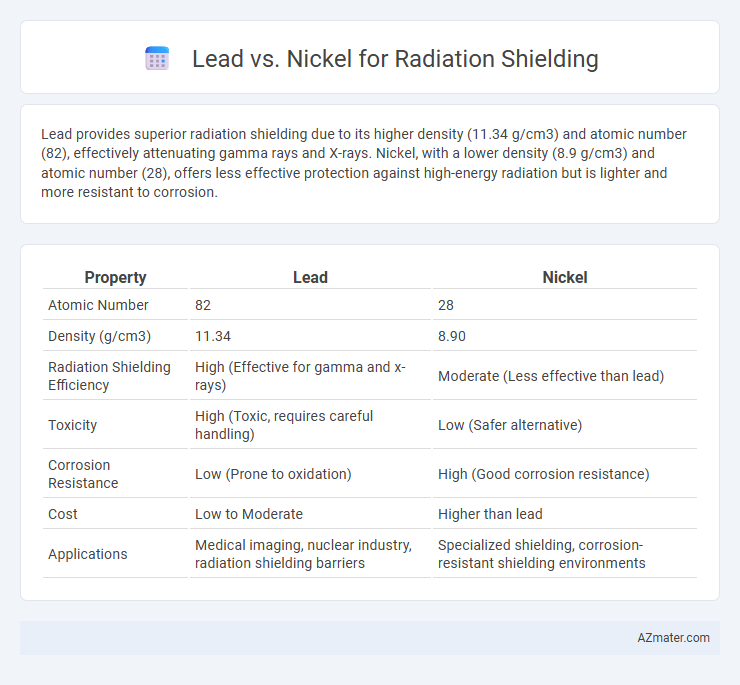
Lead provides superior radiation shielding due to its higher density (11.34 g/cm3) and atomic number (82), effectively attenuating gamma rays and X-rays. Nickel, with a lower density (8.9 g/cm3) and atomic number (28), offers less effective protection against high-energy radiation but is lighter and more resistant to corrosion.
Table of Comparison
| Property | Lead | Nickel |
|---|---|---|
| Atomic Number | 82 | 28 |
| Density (g/cm3) | 11.34 | 8.90 |
| Radiation Shielding Efficiency | High (Effective for gamma and x-rays) | Moderate (Less effective than lead) |
| Toxicity | High (Toxic, requires careful handling) | Low (Safer alternative) |
| Corrosion Resistance | Low (Prone to oxidation) | High (Good corrosion resistance) |
| Cost | Low to Moderate | Higher than lead |
| Applications | Medical imaging, nuclear industry, radiation shielding barriers | Specialized shielding, corrosion-resistant shielding environments |
Introduction to Radiation Shielding Materials
Lead and nickel are commonly utilized in radiation shielding due to their distinct physical and atomic properties. Lead's high atomic number (82) and density (11.34 g/cm3) make it highly effective in attenuating gamma and X-ray radiation. Conversely, nickel, with its lower atomic number (28) and density (8.90 g/cm3), is preferred for neutron radiation shielding and scenarios requiring corrosion resistance or structural strength.
The Role of Lead in Radiation Protection
Lead plays a crucial role in radiation protection due to its high atomic number (82) and density (11.34 g/cm3), which enable it to effectively attenuate gamma rays and X-rays. Its ability to absorb and scatter ionizing radiation makes it the preferred material for shielding in medical, industrial, and nuclear applications. While nickel offers corrosion resistance and durability, it lacks the mass density required for efficient radiation shielding, making lead the superior choice for protecting against high-energy photons.
The Use of Nickel for Radiation Shielding
Nickel offers significant advantages for radiation shielding due to its high density and excellent resistance to corrosion, making it suitable for environments exposed to radioactive materials. Its ability to effectively absorb neutron radiation complements its protective properties against gamma rays, often combined with other materials to enhance overall shielding performance. Industrial applications value nickel for durable, lightweight shielding components in nuclear reactors and medical radiation equipment.
Comparative Density and Atomic Number
Lead has a higher density of 11.34 g/cm3 compared to nickel's 8.90 g/cm3, making lead more effective for radiation shielding by providing greater mass per unit volume to attenuate radiation. The atomic number of lead is 82, significantly higher than nickel's 28, which results in greater photoelectric absorption and improved attenuation of gamma rays and X-rays. These properties make lead the preferred choice for radiation shielding in medical, industrial, and nuclear applications where maximum protection is required.
Effectiveness Against Different Types of Radiation
Lead exhibits superior effectiveness against gamma rays and X-rays due to its high density and atomic number, which enhances photon attenuation through photoelectric absorption and Compton scattering. Nickel offers moderate shielding properties, primarily effective against beta particles, but is less efficient than lead for high-energy photon radiation. For neutron radiation, neither lead nor nickel is highly effective, requiring specialized materials like boron or polyethylene for optimal protection.
Durability and Structural Considerations
Lead offers superior radiation shielding due to its high density but lacks durability, as it is soft and prone to deformation and corrosion over time. Nickel, while less effective in radiation attenuation compared to lead, provides enhanced structural integrity, resistance to wear, and greater durability in harsh environments. For applications requiring long-term mechanical stability and corrosion resistance alongside moderate radiation protection, nickel-based materials are preferred over lead.
Health and Environmental Impacts
Lead offers superior radiation shielding due to its high density but poses significant health risks such as lead poisoning and neurological damage from exposure or improper handling. Nickel, while less effective at shielding compared to lead, presents a lower toxicity profile, reducing environmental contamination and health hazards during manufacturing and disposal. Both materials require careful management, yet nickel's environmental impact is generally more favorable, making it a safer alternative in applications prioritizing sustainability and worker safety.
Cost and Availability Analysis
Lead offers superior radiation shielding due to its high density and atomic number, but it is comparatively expensive and subject to stricter environmental regulations affecting availability. Nickel, while less effective per unit thickness, provides a more cost-effective alternative with better corrosion resistance and wider material supply chains. Evaluating shielding projects requires balancing lead's superior attenuation properties against nickel's cost efficiency and easier procurement.
Practical Applications in Industry and Medicine
Lead offers superior radiation shielding due to its high density and atomic number, making it ideal for protecting against gamma rays and X-rays in medical imaging and nuclear power plants. Nickel, while less dense, provides advantages in high-temperature environments and corrosion resistance, useful in specialized industrial equipment and radiation-resistant alloys. Industries prioritize lead for cost-effective, heavy-duty protection, whereas nickel alloys serve in applications requiring durability under harsh operational conditions.
Choosing the Right Material: Lead vs Nickel
Lead offers superior radiation shielding capabilities due to its high density and atomic number, effectively attenuating gamma rays and X-rays in medical and industrial applications. Nickel, while less dense, provides durability and corrosion resistance, making it suitable for environments where mechanical strength and longevity are critical alongside moderate radiation protection. Selecting the right material depends on the specific radiation type, required shielding thickness, and environmental conditions where the shielding will be deployed.
Infographic: Lead vs Nickel for Radiation Shielding
 azmater.com
azmater.com