Osmium offers exceptional density and corrosion resistance, making it ideal for high-precision scientific instruments, while berkelium's rarity and radioactivity limit its use primarily to specialized nuclear research. Research applications prioritize osmium for stability and longevity, whereas berkelium is valuable for studying transuranic elements and nuclear reactions.
Table of Comparison
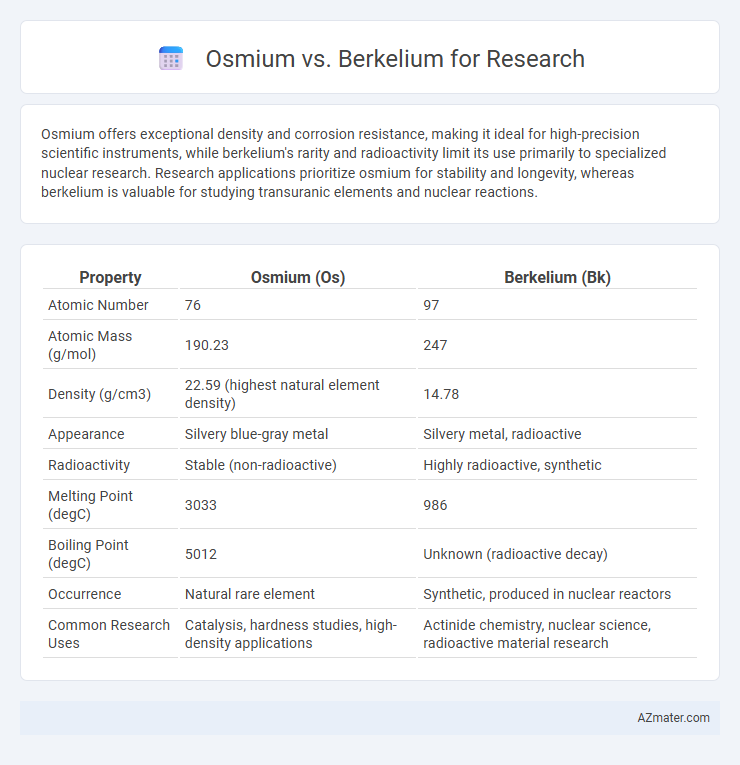
| Property | Osmium (Os) | Berkelium (Bk) |
|---|---|---|
| Atomic Number | 76 | 97 |
| Atomic Mass (g/mol) | 190.23 | 247 |
| Density (g/cm3) | 22.59 (highest natural element density) | 14.78 |
| Appearance | Silvery blue-gray metal | Silvery metal, radioactive |
| Radioactivity | Stable (non-radioactive) | Highly radioactive, synthetic |
| Melting Point (degC) | 3033 | 986 |
| Boiling Point (degC) | 5012 | Unknown (radioactive decay) |
| Occurrence | Natural rare element | Synthetic, produced in nuclear reactors |
| Common Research Uses | Catalysis, hardness studies, high-density applications | Actinide chemistry, nuclear science, radioactive material research |
Introduction to Osmium and Berkelium
Osmium, a dense transition metal with atomic number 76, is renowned for its hardness and high melting point, making it valuable in catalysis and material science research. Berkelium, a synthetic actinide with atomic number 97, is primarily used in nuclear chemistry studies and the synthesis of heavier elements due to its radioactivity and scarcity. Research involving osmium focuses on its physical and chemical stability, while berkelium studies advance understanding of actinide properties and nuclear reactions.
Elemental Properties Comparison
Osmium exhibits high density (22.59 g/cm3), exceptional hardness, and excellent corrosion resistance, making it valuable for applications requiring durability and stability in research. Berkelium, a synthetic actinide with atomic number 97, is radioactive and primarily studied for its nuclear properties and behavior in transuranic element experiments. Comparing elemental properties, osmium's stable and inert nature contrasts with berkelium's radioactivity and scarcity, influencing their roles in scientific investigations and material science research.
Abundance and Availability
Osmium, with an abundance of approximately 50 parts per billion in the Earth's crust, is relatively rare but more accessible than berkelium, which is a synthetic element produced in minute quantities through nuclear reactions. The limited availability of berkelium, primarily confined to specialized research facilities with particle accelerators, significantly restricts its use in experimentation compared to osmium's wider albeit still limited supply. Osmium's greater abundance and commercial availability make it a more practical choice for extensive scientific research, whereas berkelium remains valuable mainly for niche studies in nuclear science and advanced materials.
Synthesis and Isolation Methods
Osmium is synthesized primarily through the refinement of platinum ores containing osmium-iridium alloys, involving high-temperature dissolution in aqua regia and subsequent fractional crystallization to isolate pure osmium. Berkelium, a synthetic actinide element, is produced by neutron irradiation of americium-241 targets in nuclear reactors, followed by complex solvent extraction and ion-exchange chromatography for purification. The isolation of osmium relies on its chemical inertness and density, while berkelium's separation depends on its radioactive decay properties and coordination chemistry in highly controlled radiochemical facilities.
Chemical Reactivity and Behavior
Osmium, a dense transition metal with atomic number 76, exhibits low chemical reactivity, primarily forming stable oxidation states such as +2, +3, and +4, making it valuable in catalytic and electrochemical research. Berkelium, an actinide with atomic number 97, is highly radioactive and exhibits complex chemical behavior with variable oxidation states (+3 and +4), making its chemical reactivity less predictable and challenging in experimental studies. Research comparing osmium and berkelium emphasizes osmium's stability and catalytic potential against berkelium's unique radioactive properties and complex electron configurations impacting its reactivity.
Applications in Scientific Research
Osmium, known for its extreme density and hardness, finds applications in scientific research areas such as electron microscopy, where its use as an electron-dense staining agent enhances imaging resolution. Berkelium, a synthetic actinide with limited availability, is primarily utilized in nuclear science research to study transuranic elements and synthesize heavier elements through nuclear reactions. The contrasting properties and availability of osmium and berkelium define their distinct roles in advancing materials science and nuclear chemistry, respectively.
Safety and Handling Considerations
Osmium is dense and relatively inert but produces toxic osmium tetroxide upon oxidation, requiring strict ventilation and protective gear during handling. Berkelium, a radioactive actinide, demands rigorous radiological safety protocols, including remote handling and specialized containment to prevent contamination. Both elements necessitate controlled laboratory environments to mitigate chemical toxicity and radiological hazards in research applications.
Economic and Environmental Impact
Osmium offers significant economic advantages in research due to its rarity, high market value, and applications in precision instruments and catalysts, contributing to cost-intensive but high-impact studies. Berkelium, primarily used in nuclear research, incurs substantial environmental risks from radioactive waste management and necessitates stringent safety protocols, increasing overall research costs. The environmental footprint of osmium extraction is lower compared to the radiological hazards and disposal challenges associated with berkelium, making osmium a more economically and environmentally viable choice for many research applications.
Recent Advances and Discoveries
Recent advances in osmium research highlight its exceptional catalytic properties and high-density applications in material science, enabling breakthroughs in precision instruments and cancer treatment methods. Berkelium studies have progressed with improved synthesis techniques, facilitating its use in nuclear science for producing heavier elements and understanding actinide behavior. These discoveries position osmium and berkelium as critical elements for advancing nanotechnology and nuclear chemistry research.
Future Research Prospects
Osmium's exceptional density and stability make it a prime candidate for advanced material science research, including applications in high-precision instruments and catalysis. Berkelium's radioactive properties and scarcity pose challenges but also open avenues in nuclear science, particularly in synthesizing heavier elements and studying actinide chemistry. Future research will likely prioritize osmium for practical technological innovations, while berkelium remains critical for expanding the understanding of nuclear reactions and element formation.
Infographic: Osmium vs Berkelium for Research
 azmater.com
azmater.com