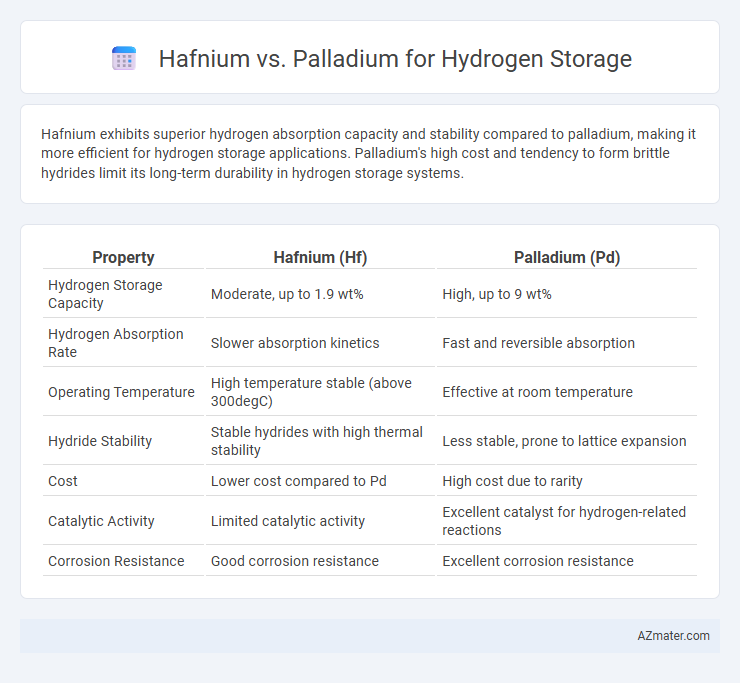
Hafnium exhibits superior hydrogen absorption capacity and stability compared to palladium, making it more efficient for hydrogen storage applications. Palladium's high cost and tendency to form brittle hydrides limit its long-term durability in hydrogen storage systems.
Table of Comparison
| Property | Hafnium (Hf) | Palladium (Pd) |
|---|---|---|
| Hydrogen Storage Capacity | Moderate, up to 1.9 wt% | High, up to 9 wt% |
| Hydrogen Absorption Rate | Slower absorption kinetics | Fast and reversible absorption |
| Operating Temperature | High temperature stable (above 300degC) | Effective at room temperature |
| Hydride Stability | Stable hydrides with high thermal stability | Less stable, prone to lattice expansion |
| Cost | Lower cost compared to Pd | High cost due to rarity |
| Catalytic Activity | Limited catalytic activity | Excellent catalyst for hydrogen-related reactions |
| Corrosion Resistance | Good corrosion resistance | Excellent corrosion resistance |
Introduction to Hydrogen Storage Materials
Hydrogen storage materials are critical for enabling efficient energy systems, with metals like hafnium and palladium offering distinct advantages due to their unique physicochemical properties. Hafnium exhibits high hydrogen absorption capacity and stability, making it favorable for long-term storage applications, while palladium is renowned for its exceptional hydrogen solubility and rapid absorption kinetics. Understanding the comparative hydrogen storage performance of these metals is essential for advancing sustainable energy technologies.
Overview of Hafnium as a Hydrogen Storage Medium
Hafnium exhibits strong potential as a hydrogen storage medium due to its high hydrogen absorption capacity and excellent thermal stability, making it suitable for high-temperature applications. Its ability to form stable hydrides enables efficient hydrogen release and uptake cycles, with a hydrogen-to-metal ratio reaching up to 1.9 under optimal conditions. Compared to palladium, hafnium offers lower material costs and improved resistance to hydrogen embrittlement, which enhances durability for long-term hydrogen storage systems.
Palladium’s Role in Hydrogen Storage Technologies
Palladium exhibits exceptional hydrogen absorption capabilities, making it a cornerstone in hydrogen storage technologies due to its ability to reversibly absorb and release hydrogen at room temperature. Its high permeability to hydrogen molecules facilitates efficient storage and rapid hydrogen cycling, which is critical for fuel cell applications. Compared to hafnium, palladium offers superior catalytic properties and a well-established infrastructure for integration into practical hydrogen storage systems.
Hydrogen Absorption Mechanisms in Hafnium
Hafnium exhibits exceptional hydrogen absorption capabilities due to its hexagonal close-packed crystal structure, which facilitates efficient hydrogen diffusion and storage through the formation of stable hydride phases. The hydrogen absorption mechanism in hafnium primarily involves the dissociation of molecular hydrogen on the metal surface followed by atomic hydrogen diffusion into interstitial sites, promoting reversible hydrogen storage. This behavior contrasts with palladium's face-centered cubic lattice, where hydrogen absorption is dominated by rapid surface adsorption and subsurface diffusion but often suffers from lattice expansion-induced stress and limited hydride stability.
Hydrogen Absorption Mechanisms in Palladium
Palladium exhibits exceptional hydrogen absorption due to its unique ability to dissociate hydrogen molecules into atoms that diffuse rapidly into its lattice, forming a metal hydride with high storage capacity. This mechanism relies on palladium's face-centered cubic (FCC) crystal structure, which accommodates hydrogen atoms interstitially, leading to reversible absorption and desorption cycles essential for efficient hydrogen storage. In contrast, hafnium's hydrogen absorption is less efficient due to its hexagonal close-packed (HCP) structure, which limits hydrogen solubility and diffusion rates compared to palladium.
Comparative Storage Capacity: Hafnium vs Palladium
Hafnium exhibits a high hydrogen storage capacity due to its strong affinity for hydrogen, forming stable hydrides with significant volumetric density. Palladium is widely recognized for its exceptional ability to absorb hydrogen at the atomic level, offering rapid kinetics but with lower overall storage capacity than hafnium. Comparative studies reveal hafnium surpasses palladium in total hydrogen storage capacity, making it a promising candidate for applications requiring high-density hydrogen containment.
Kinetic Performance and Cycling Stability
Hafnium exhibits superior kinetic performance in hydrogen storage due to its faster absorption and desorption rates compared to palladium, which often suffers from slower hydrogen uptake. Palladium, while highly efficient in hydrogen adsorption, tends to degrade over multiple hydrogenation cycles, leading to reduced cycling stability. Hafnium alloys demonstrate enhanced cycling stability with minimal capacity loss over repeated hydrogen absorption and desorption cycles, making them more durable for long-term hydrogen storage applications.
Cost and Availability of Hafnium and Palladium
Hafnium is significantly less abundant and more expensive than palladium due to its limited supply as a byproduct of zirconium refining, impacting its feasibility for large-scale hydrogen storage applications. Palladium, while also costly, benefits from higher availability and well-established extraction and recycling processes, making it more economically viable for hydrogen storage technologies. The cost disparity and supply constraints make palladium a more practical choice compared to hafnium for efficient and scalable hydrogen storage solutions.
Safety and Environmental Considerations
Hafnium exhibits superior safety in hydrogen storage due to its high resistance to embrittlement and stable hydride formation, minimizing risks of material failure and hydrogen leakage. Palladium, while highly effective in hydrogen absorption, poses environmental concerns related to its limited availability and mining impact, alongside potential toxicity in catalytic waste. The environmental footprint and durability of hafnium make it a safer, more sustainable choice for long-term hydrogen storage applications.
Future Prospects and Research Directions
Hafnium exhibits promising hydrogen storage capabilities due to its high absorption capacity and stability under varying conditions, making it a focus for advanced energy applications. Palladium remains a benchmark material thanks to its exceptional hydrogen permeability and reversible adsorption, though cost and weight limit large-scale use. Emerging research aims to enhance hafnium alloys for improved kinetics and develop hybrid palladium-based composites to balance performance and economic feasibility in next-generation hydrogen storage systems.
Infographic: Hafnium vs Palladium for Hydrogen Storage
 azmater.com
azmater.com