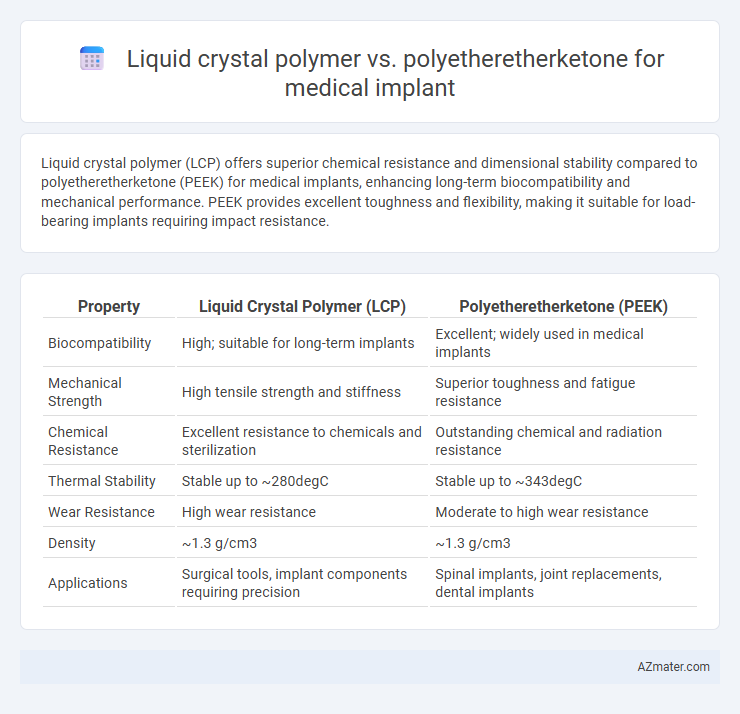
Liquid crystal polymer (LCP) offers superior chemical resistance and dimensional stability compared to polyetheretherketone (PEEK) for medical implants, enhancing long-term biocompatibility and mechanical performance. PEEK provides excellent toughness and flexibility, making it suitable for load-bearing implants requiring impact resistance.
Table of Comparison
| Property | Liquid Crystal Polymer (LCP) | Polyetheretherketone (PEEK) |
|---|---|---|
| Biocompatibility | High; suitable for long-term implants | Excellent; widely used in medical implants |
| Mechanical Strength | High tensile strength and stiffness | Superior toughness and fatigue resistance |
| Chemical Resistance | Excellent resistance to chemicals and sterilization | Outstanding chemical and radiation resistance |
| Thermal Stability | Stable up to ~280degC | Stable up to ~343degC |
| Wear Resistance | High wear resistance | Moderate to high wear resistance |
| Density | ~1.3 g/cm3 | ~1.3 g/cm3 |
| Applications | Surgical tools, implant components requiring precision | Spinal implants, joint replacements, dental implants |
Introduction to Liquid Crystal Polymer (LCP) and Polyetheretherketone (PEEK)
Liquid Crystal Polymer (LCP) exhibits exceptional chemical resistance, dimensional stability, and biocompatibility, making it suitable for intricate medical implant components requiring precision and durability. Polyetheretherketone (PEEK) offers high mechanical strength, excellent wear resistance, and radiolucency, widely utilized in load-bearing orthopedic and spinal implants. Both polymers are favored for their sterilization tolerance and compatibility with long-term implantation, but LCP is particularly advantageous in micro-scale applications while PEEK dominates structural implant uses.
Material Composition and Structural Differences
Liquid crystal polymer (LCP) consists of aromatic polyester chains that align in a highly ordered, crystalline structure, offering exceptional tensile strength and chemical resistance ideal for medical implants. Polyetheretherketone (PEEK) is a semi-crystalline thermoplastic composed of repeated ether and ketone linkages, providing excellent mechanical stability, biocompatibility, and resistance to body fluids. The structural difference lies in LCP's rigid, rod-like molecular arrangement compared to PEEK's more flexible chain structure, influencing their respective performance in implant durability and integration.
Biocompatibility of LCP vs PEEK in Medical Implants
Liquid crystal polymer (LCP) exhibits superior biocompatibility compared to polyetheretherketone (PEEK) in medical implants due to its excellent chemical inertness and minimal tissue response, reducing inflammation and fibrosis risks. LCP's low moisture absorption and resistance to bacterial colonization enhance implant longevity and patient safety. In contrast, PEEK, while biocompatible, often requires surface modifications to improve osseointegration and reduce biofilm formation in clinical applications.
Mechanical Properties Comparison: Strength, Flexibility, and Fatigue
Liquid crystal polymer (LCP) exhibits superior tensile strength and excellent chemical resistance compared to polyetheretherketone (PEEK), making it highly suitable for high-stress medical implants. PEEK offers greater flexibility and impact resistance, enhancing its performance in load-bearing applications where deformation without failure is critical. Fatigue resistance in PEEK generally surpasses LCP, ensuring longer durability in cyclic loading environments common in orthopedic and dental implants.
Chemical Resistance and Durability in Biological Environments
Liquid crystal polymer (LCP) exhibits exceptional chemical resistance, maintaining stability against bodily fluids and sterilization chemicals, making it highly suitable for long-term medical implants. Polyetheretherketone (PEEK) offers superior durability with excellent mechanical strength and fatigue resistance under physiological conditions, ensuring reliable performance in dynamic biological environments. Both materials resist hydrolysis, but LCP's low permeability and PEEK's biocompatibility optimize implant longevity and chemical stability in vivo.
Radiolucency and Imaging Compatibility
Liquid crystal polymer (LCP) offers superior radiolucency compared to polyetheretherketone (PEEK), enabling clearer imaging results during medical implant evaluations. LCP's minimal interference with X-rays and MRI scans enhances diagnostic accuracy and allows for more precise postoperative assessments. In contrast, PEEK, while biocompatible and mechanically robust, exhibits lower radiolucency, potentially obscuring implant visualization in imaging procedures.
Sterilization Methods and Their Effects on LCP and PEEK
Liquid crystal polymer (LCP) and polyetheretherketone (PEEK) exhibit distinct responses to sterilization methods commonly used for medical implants, such as autoclaving, gamma irradiation, and ethylene oxide exposure. LCP maintains superior dimensional stability and mechanical strength after gamma irradiation but can experience embrittlement under high-temperature autoclaving, whereas PEEK demonstrates excellent resistance to autoclaving with minimal degradation in tensile properties and chemical structure. Understanding these material-specific effects enables optimized implant performance and longevity by selecting appropriate sterilization techniques tailored to LCP's sensitivity to heat and PEEK's robustness under varied sterilization conditions.
Fabrication Techniques and Design Flexibility
Liquid crystal polymer (LCP) offers exceptional precision in microfabrication processes such as injection molding and laser micromachining, enabling highly intricate medical implant designs with superior chemical resistance and biocompatibility. Polyetheretherketone (PEEK) supports advanced fabrication methods including CNC machining, 3D printing, and compression molding, providing enhanced design flexibility for customized, load-bearing orthopedic implants due to its high mechanical strength and thermal stability. The choice between LCP and PEEK significantly impacts the achievable complexity and performance of medical implants, with LCP excelling in micro-scale precision and PEEK offering broader adaptability for complex geometries and functional gradients.
Clinical Performance and Case Studies
Liquid crystal polymer (LCP) exhibits exceptional biocompatibility, high tensile strength, and chemical resistance, making it highly suitable for durable medical implants in cardiovascular and orthopedic applications. Polyetheretherketone (PEEK) demonstrates superior mechanical stability, radiolucency, and resistance to wear, widely validated in spinal and cranial implant case studies with favorable osseointegration and infection resistance. Clinical performance comparisons highlight LCP's flexibility and fatigue resistance for dynamic implant sites, whereas PEEK offers robust structural support and proven long-term outcomes in load-bearing implants.
Cost Analysis and Future Prospects for LCP and PEEK in Medical Implants
Liquid crystal polymer (LCP) generally incurs higher initial costs than polyetheretherketone (PEEK) due to complex manufacturing and material purity requirements, but LCP offers superior chemical resistance and lower moisture absorption, making it ideal for long-term implants. PEEK is favored for its cost-effectiveness, excellent mechanical strength, and biocompatibility, supporting widespread use in orthopedic and spinal implants. Future prospects indicate growing demand for LCP in high-performance, miniaturized implants while PEEK innovations focus on enhanced bioactivity and cost reduction to maintain market dominance in medical device applications.
Infographic: Liquid crystal polymer vs Polyetheretherketone for Medical Implant
 azmater.com
azmater.com