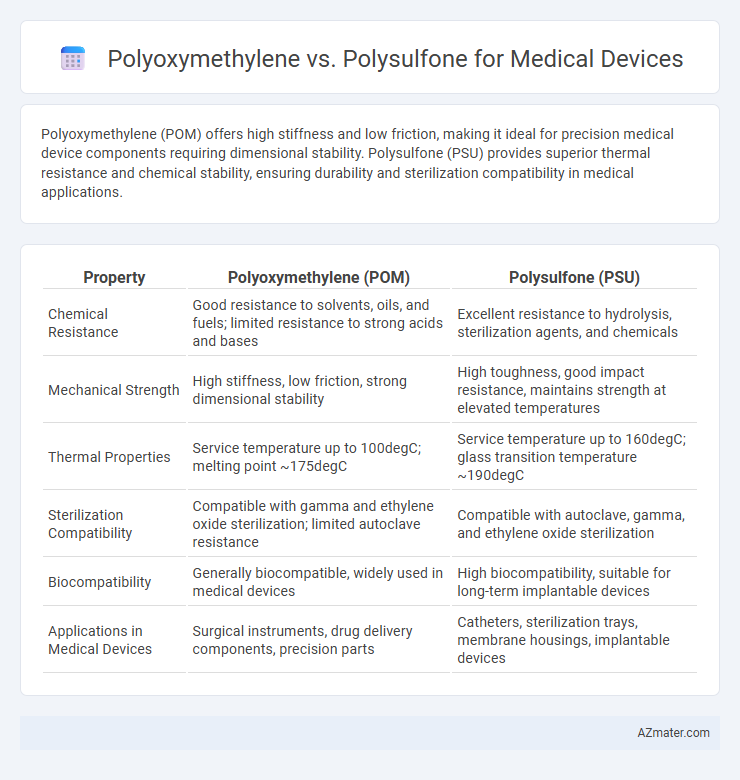
Polyoxymethylene (POM) offers high stiffness and low friction, making it ideal for precision medical device components requiring dimensional stability. Polysulfone (PSU) provides superior thermal resistance and chemical stability, ensuring durability and sterilization compatibility in medical applications.
Table of Comparison
| Property | Polyoxymethylene (POM) | Polysulfone (PSU) |
|---|---|---|
| Chemical Resistance | Good resistance to solvents, oils, and fuels; limited resistance to strong acids and bases | Excellent resistance to hydrolysis, sterilization agents, and chemicals |
| Mechanical Strength | High stiffness, low friction, strong dimensional stability | High toughness, good impact resistance, maintains strength at elevated temperatures |
| Thermal Properties | Service temperature up to 100degC; melting point ~175degC | Service temperature up to 160degC; glass transition temperature ~190degC |
| Sterilization Compatibility | Compatible with gamma and ethylene oxide sterilization; limited autoclave resistance | Compatible with autoclave, gamma, and ethylene oxide sterilization |
| Biocompatibility | Generally biocompatible, widely used in medical devices | High biocompatibility, suitable for long-term implantable devices |
| Applications in Medical Devices | Surgical instruments, drug delivery components, precision parts | Catheters, sterilization trays, membrane housings, implantable devices |
Introduction to Polyoxymethylene and Polysulfone in Medical Devices
Polyoxymethylene (POM) is a high-performance engineering thermoplastic known for its excellent mechanical strength, low friction, and chemical resistance, making it ideal for precision parts in medical devices such as surgical instruments and drug delivery systems. Polysulfone (PSU) offers superior thermal stability, biocompatibility, and resistance to steam sterilization, frequently used in applications like fluid handling components and sterilizable medical housings. Both materials provide critical properties for medical device manufacturing, with POM favored for its durability and PSU chosen for high-temperature and sterilization environments.
Chemical Composition and Material Structure
Polyoxymethylene (POM) is a semicrystalline polymer composed of repeat units of formaldehyde, offering high crystallinity that provides excellent tensile strength, rigidity, and chemical resistance suitable for medical device components. Polysulfone (PSU) is an amorphous thermoplastic characterized by repeating sulfone and aromatic units, granting superior thermal stability, hydrolytic resistance, and biocompatibility critical for sterilizable medical devices. The structural differences between POM's linear, crystalline chains and PSU's rigid, non-crystalline aromatic backbone influence their chemical resistance and mechanical performance under medical sterilization conditions.
Mechanical Strength and Durability Comparison
Polyoxymethylene (POM) offers high mechanical strength with excellent fatigue resistance, making it suitable for components requiring rigidity and dimensional stability in medical devices. Polysulfone (PSU) excels in durability with superior impact resistance and maintains structural integrity under sterilization processes involving heat and chemicals. PSU's enhanced thermal stability and toughness outperform POM in long-term applications requiring frequent sterilization cycles.
Thermal Stability and Heat Resistance
Polyoxymethylene (POM) offers excellent mechanical properties but has limited thermal stability, typically with a melting point around 175degC and a lower continuous service temperature, which can restrict its use in high-heat medical device applications. Polysulfone (PSU) exhibits superior heat resistance with a continuous service temperature up to 160-190degC and exceptional thermal stability, making it ideal for sterilization processes such as autoclaving and repeated high-temperature exposures. For medical devices requiring durability under high thermal stress, polysulfone outperforms polyoxymethylene due to its resistance to thermal degradation and dimensional stability at elevated temperatures.
Biocompatibility and Safety for Medical Use
Polyoxymethylene (POM) offers excellent mechanical strength and chemical resistance, making it suitable for precise medical components, but it has limited biocompatibility and can release formaldehyde under certain conditions, posing potential safety concerns. Polysulfone (PSU) exhibits superior biocompatibility with high resistance to heat, steam, and sterilization processes, making it ideal for long-term medical devices requiring repeated sterilization without degradation. Medical applications prioritize polysulfone for implants and surgical instruments due to its proven safety profile and minimal cytotoxicity compared to polyoxymethylene.
Resistance to Chemicals and Sterilization Methods
Polyoxymethylene (POM) offers excellent resistance to aqueous chemicals and is compatible with common sterilization methods such as ethylene oxide and gamma radiation, making it suitable for many medical device components. Polysulfone (PSU) exhibits superior resistance to harsh chemicals, including oxidizing agents and solvents, and maintains mechanical integrity after repeated steam sterilization (autoclaving), essential for reusable medical devices. When selecting materials for medical devices requiring frequent sterilization and exposure to aggressive chemicals, polysulfone provides enhanced durability, while polyoxymethylene is preferable for applications with milder chemical exposure.
Applications in Medical Device Manufacturing
Polyoxymethylene (POM) is widely used in medical device manufacturing for precision components requiring high stiffness, low friction, and excellent dimensional stability, such as surgical instrument handles and fluid connectors. Polysulfone (PSU) excels in applications demanding superior chemical resistance, high temperature tolerance, and biocompatibility, making it ideal for sterilizable equipment components, membranes, and implantable device housings. Both polymers are selected based on the specific mechanical, thermal, and chemical requirements crucial for ensuring device safety and performance in medical environments.
Cost-Effectiveness and Economic Considerations
Polyoxymethylene (POM) offers superior cost-effectiveness compared to Polysulfone (PSU) due to its lower raw material and processing costs, making it ideal for high-volume medical device production. While Polysulfone provides excellent thermal stability and chemical resistance, its higher price point can impact budget constraints in large-scale manufacturing. Evaluating the total cost of ownership, including tooling, machining, and sterilization expenses, is crucial when choosing between POM and PSU for economically viable medical devices.
Regulatory Compliance and Certification Standards
Polyoxymethylene (POM) and Polysulfone (PSU) are widely used polymers in medical device manufacturing, with Polysulfone offering superior compliance with ISO 10993 biocompatibility standards and USP Class VI certification. POM demonstrates strong mechanical properties but may face limitations in sterilization methods due to lower thermal stability compared to Polysulfone, which withstands steam sterilization (autoclaving) and gamma radiation effectively. Regulatory approval for devices using Polysulfone is streamlined by its established history in FDA 510(k) clearances and CE marking compliance, making it preferable for critical medical components requiring rigorous certification.
Choosing the Right Material: Key Factors and Recommendations
Polyoxymethylene (POM) offers excellent rigidity, low moisture absorption, and high dimensional stability, making it ideal for precision medical components like housings and connectors. Polysulfone (PSU) provides superior thermal resistance, chemical durability, and biocompatibility, suitable for sterilizable devices and fluid-handling parts. Selecting between POM and PSU depends on the specific device requirements for temperature tolerance, chemical exposure, mechanical strength, and regulatory compliance under ISO 10993 standards.
Infographic: Polyoxymethylene vs Polysulfone for Medical device
 azmater.com
azmater.com