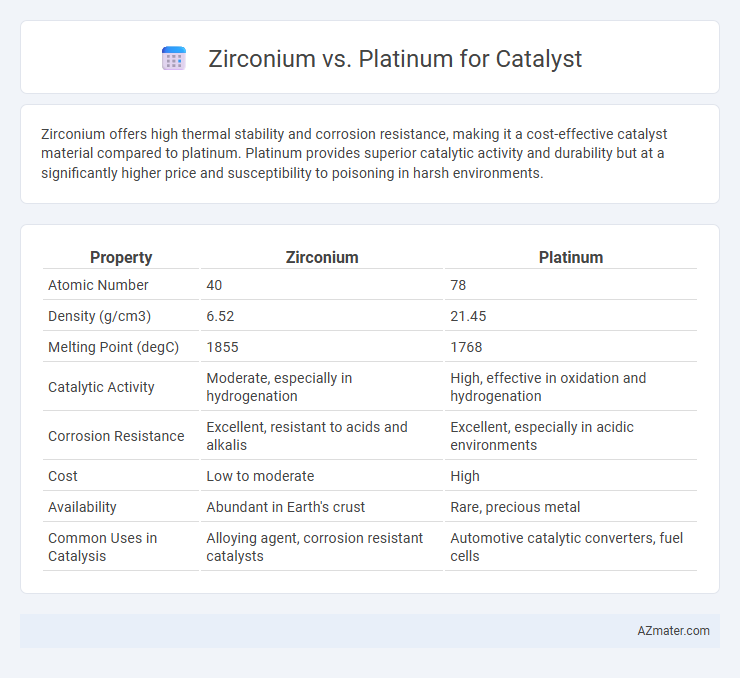
Zirconium offers high thermal stability and corrosion resistance, making it a cost-effective catalyst material compared to platinum. Platinum provides superior catalytic activity and durability but at a significantly higher price and susceptibility to poisoning in harsh environments.
Table of Comparison
| Property | Zirconium | Platinum |
|---|---|---|
| Atomic Number | 40 | 78 |
| Density (g/cm3) | 6.52 | 21.45 |
| Melting Point (degC) | 1855 | 1768 |
| Catalytic Activity | Moderate, especially in hydrogenation | High, effective in oxidation and hydrogenation |
| Corrosion Resistance | Excellent, resistant to acids and alkalis | Excellent, especially in acidic environments |
| Cost | Low to moderate | High |
| Availability | Abundant in Earth's crust | Rare, precious metal |
| Common Uses in Catalysis | Alloying agent, corrosion resistant catalysts | Automotive catalytic converters, fuel cells |
Introduction to Catalyst Materials: Zirconium vs Platinum
Zirconium and platinum are critical materials in catalyst design, each offering distinct advantages in chemical reactions. Platinum serves as a highly efficient catalyst due to its exceptional electronic properties and resistance to poisoning, making it ideal for automotive catalytic converters and fuel cells. Zirconium, often used in oxide form, provides thermal stability and supports active catalyst phases, enhancing durability and catalytic performance in harsh industrial environments.
Chemical Properties: Zirconium and Platinum as Catalysts
Zirconium exhibits high thermal stability and resistance to corrosion, making it effective for catalytic processes in harsh chemical environments. Platinum offers exceptional catalytic activity and selectivity, especially in hydrogenation and oxidation reactions, due to its ability to adsorb and activate molecules at the atomic level. Zirconium's oxophilic nature contrasts with platinum's noble metal characteristics, influencing their roles in oxygen-dependent versus hydrogen-rich catalytic applications.
Catalytic Efficiency: Performance Comparison
Zirconium-based catalysts demonstrate high thermal stability and enhanced oxygen ion conductivity, making them efficient in oxidation and reforming reactions, especially in automotive and fuel cell applications. Platinum catalysts exhibit superior catalytic activity and selectivity, particularly in hydrogenation and dehydrogenation processes, due to their excellent electronic properties and ability to adsorb reactants effectively. While platinum catalysts generally offer higher catalytic efficiency in low-temperature reactions, zirconium's robust performance under harsh conditions offers a cost-effective alternative in high-temperature and large-scale industrial processes.
Cost Analysis: Zirconium vs Platinum Catalysts
Zirconium catalysts offer a significant cost advantage over platinum catalysts due to the lower raw material and extraction expenses. Platinum's high market price and scarcity contribute to elevated catalyst costs, making zirconium a more economically feasible option for large-scale applications. The durability and efficiency of zirconium catalysts can further reduce long-term operational costs compared to platinum-based alternatives.
Durability and Stability in Catalytic Reactions
Zirconium-based catalysts demonstrate exceptional durability due to their high resistance to thermal degradation and chemical corrosion, making them ideal for harsh catalytic environments. Platinum catalysts offer superior catalytic activity but may suffer from agglomeration and sintering, reducing their stability over prolonged reactions. Zirconium's ability to maintain structural integrity under extreme conditions enhances long-term catalytic performance compared to platinum.
Industrial Applications: Where Zirconium and Platinum Excel
Zirconium excels in industrial catalytic applications requiring strong corrosion resistance and thermal stability, such as in petrochemical processing and nuclear reactors. Platinum is preferred for automotive catalytic converters and chemical synthesis due to its exceptional catalytic activity and durability under harsh reaction conditions. Both metals play critical roles in industry, with zirconium favored for structural catalyst supports and platinum for active surface catalysts in fuel cells and pollution control.
Environmental Impact of Zirconium and Platinum Catalysts
Zirconium catalysts generally exhibit lower environmental toxicity and greater abundance compared to platinum, which is a rare metal with significant extraction-related environmental impacts. The mining and refining of platinum generate substantial greenhouse gas emissions and hazardous waste, whereas zirconium extraction tends to have a smaller ecological footprint. Catalysts made from zirconium also offer enhanced recyclability and reduced ecological disruption, making them a more sustainable option in industrial applications.
Availability and Sourcing of Catalyst Materials
Zirconium is more abundant in the Earth's crust, making it relatively easier and cost-effective to source compared to platinum, which is a rare and precious metal primarily mined in limited regions such as South Africa and Russia. The widespread availability of zirconium compounds supports large-scale catalyst production with stable supply chains, whereas platinum's scarcity and geopolitical concentration result in higher volatility in availability and pricing. Consequently, zirconium-based catalysts offer a more sustainable and secure option for industrial applications requiring reliable sourcing of catalytic materials.
Future Trends in Catalyst Development
Zirconium-based catalysts are gaining attention in future catalyst development due to their superior thermal stability and affordability compared to platinum. Emerging research highlights zirconium's potential for enhancing catalytic efficiency in green hydrogen production and CO2 reduction technologies. Platinum remains critical for high-performance applications but faces challenges related to scarcity and cost, driving innovation toward zirconium alloy catalysts for sustainable and scalable solutions.
Conclusion: Choosing Between Zirconium and Platinum for Catalysis
Zirconium catalysts offer cost-effective and corrosion-resistant alternatives with excellent thermal stability, making them ideal for sustainable and large-scale applications. Platinum catalysts demonstrate superior activity and selectivity, especially in hydrogenation and oxidation reactions, but their high cost and susceptibility to poisoning limit widespread use. Selecting between zirconium and platinum hinges on balancing economic constraints, catalytic performance, and specific reaction requirements to optimize process efficiency.
Infographic: Zirconium vs Platinum for Catalyst
 azmater.com
azmater.com