Osmium, a dense and corrosion-resistant metal, is not commonly used as nuclear fuel due to its lack of fissile isotopes, unlike plutonium, which is a critical fissile material extensively utilized in nuclear reactors and weapons. Plutonium-239's ability to sustain a nuclear chain reaction makes it essential for energy generation and nuclear applications.
Table of Comparison
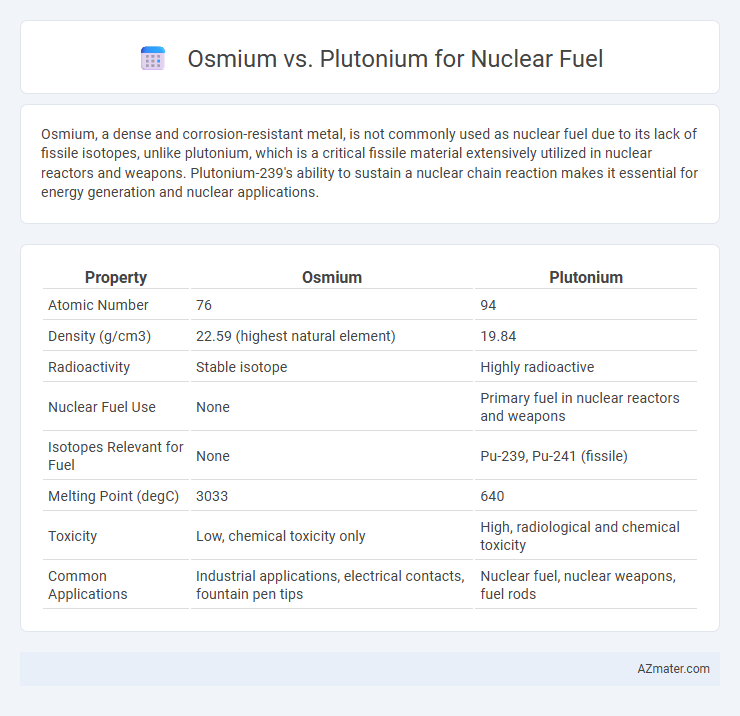
| Property | Osmium | Plutonium |
|---|---|---|
| Atomic Number | 76 | 94 |
| Density (g/cm3) | 22.59 (highest natural element) | 19.84 |
| Radioactivity | Stable isotope | Highly radioactive |
| Nuclear Fuel Use | None | Primary fuel in nuclear reactors and weapons |
| Isotopes Relevant for Fuel | None | Pu-239, Pu-241 (fissile) |
| Melting Point (degC) | 3033 | 640 |
| Toxicity | Low, chemical toxicity only | High, radiological and chemical toxicity |
| Common Applications | Industrial applications, electrical contacts, fountain pen tips | Nuclear fuel, nuclear weapons, fuel rods |
Introduction to Nuclear Fuels: Osmium vs Plutonium
Plutonium serves as a critical nuclear fuel due to its fissile properties, enabling sustained chain reactions in reactors, while osmium, despite its high density and stability, lacks the necessary nuclear characteristics for fission. Plutonium-239's ability to undergo induced fission efficiently contrasts with osmium's inert nuclear behavior, making it unsuitable for energy generation in nuclear reactors. The role of plutonium in nuclear fuel cycles, particularly in breeder reactors, underscores its importance compared to osmium's limited application in nuclear technology.
Elemental Properties Relevant to Nuclear Energy
Osmium, a dense transition metal with a high atomic number (76), exhibits significant neutron absorption properties but lacks fissile isotopes required for sustained nuclear reactions, making it unsuitable as nuclear fuel. Plutonium, specifically isotope Pu-239, possesses a favorable half-life and fissile qualities critical for chain reactions in nuclear reactors and weapons. The key elemental differences center on plutonium's ability to undergo fission efficiently, whereas osmium's nuclear properties are not conducive to energy generation or fuel cycle applications.
Abundance and Availability in Nature
Osmium is extremely rare in the Earth's crust, with an average abundance of about 0.001 parts per million, primarily found in platinum ores and difficult to extract in significant quantities. Plutonium is not naturally abundant and is primarily produced artificially in nuclear reactors through neutron capture by uranium-238. The natural scarcity of both elements limits their direct availability as nuclear fuel, but plutonium's production via uranium recycling makes it more accessible for nuclear applications.
Nuclear Fission Characteristics
Osmium, a dense transition metal, has limited potential as nuclear fuel due to its low neutron absorption cross-section and lack of fissile isotopes, making it unsuitable for sustaining nuclear fission chains. Plutonium, particularly isotope Pu-239, exhibits a high fission cross-section and emits neutrons efficiently, enabling a sustained and controlled chain reaction essential for nuclear reactors and weapons. The fission characteristics of plutonium include high energy release and the ability to breed new fissile material, whereas osmium's nuclear properties do not support these critical aspects of efficient nuclear fuel.
Energy Yield Comparison
Osmium, a dense transition metal, is not suitable for nuclear fuel due to its stable isotopes and lack of fissile properties, whereas plutonium, particularly Plutonium-239, is a highly efficient fissile material used extensively in nuclear reactors and weapons. Plutonium-239 exhibits a high energy yield of approximately 210 MeV per fission event, enabling substantial energy generation from small quantities, while osmium cannot sustain a nuclear chain reaction. The superior fission characteristics and energy output of plutonium make it the preferred choice in nuclear fuel applications compared to osmium.
Safety and Radiation Profiles
Osmium is a dense transition metal with low radioactivity, making it inherently safer to handle compared to plutonium, which is highly radioactive and poses significant radiological hazards due to its alpha particle emissions. Plutonium's use in nuclear fuel is well-established because of its fissile properties, but it requires stringent containment and shielding to prevent radiation exposure and environmental contamination. In contrast, osmium lacks fissile capabilities, limiting its application in nuclear fuel, and its safety profile is primarily related to chemical toxicity rather than radiation risk.
Handling and Storage Requirements
Osmium, a dense and rare transition metal, poses significant challenges in handling due to its high toxicity and potential to form volatile osmium tetroxide, requiring specialized containment and ventilation systems. Plutonium, a radioactive actinide used extensively in nuclear fuel, demands stringent radiological control measures, secure storage in shielded and corrosion-resistant containers, and continuous monitoring to prevent criticality and contamination. Both materials necessitate rigorous safety protocols, but plutonium's radiological hazards make its handling and storage more complex and heavily regulated.
Fuel Cycle Efficiency
Osmium is not commonly used as a nuclear fuel due to its high density but lack of fissile isotopes, whereas plutonium, particularly Pu-239, is a key component in nuclear fuel cycles for its ability to sustain chain reactions. Plutonium enables efficient fuel cycle management through reprocessing and recycling in mixed oxide (MOX) fuel, enhancing overall fuel utilization and reducing waste. The closed fuel cycle involving plutonium maximizes energy extraction and lowers the demand for natural uranium compared to traditional once-through cycles.
Environmental Impact and Waste Management
Osmium, a dense and rare transition metal, has limited applications in nuclear fuel compared to plutonium, which is a key element in nuclear reactors and weapons. Plutonium's high radioactivity and long half-life result in significant environmental hazards, requiring complex containment and long-term waste management strategies to mitigate radioactive contamination. In contrast, osmium's environmental impact is mainly due to its toxicity in chemical form rather than radioactivity, making its waste management challenges less severe but still critical for environmental safety.
Future Prospects and Research Directions
Osmium, a dense transition metal with high corrosion resistance, remains largely unexplored as a nuclear fuel compared to plutonium, which is well-established in nuclear reactors and weapons due to its fissile isotopes like Pu-239. Future prospects for nuclear fuel research emphasize improving plutonium handling and recycling technologies to reduce radioactive waste and enhance sustainability, while osmium's potential lies in its unique nuclear properties that could support advanced fuel designs or radiation shielding. Ongoing research focuses on isotopic behavior under neutron flux, fuel cycle integration, and material stability to unlock innovative applications in next-generation nuclear systems.
Infographic: Osmium vs Plutonium for Nuclear Fuel
 azmater.com
azmater.com