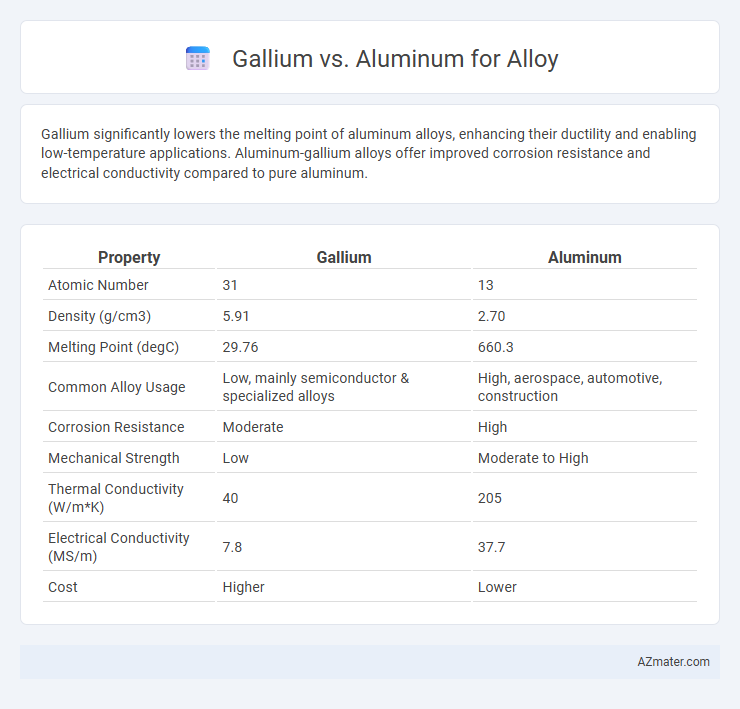
Gallium significantly lowers the melting point of aluminum alloys, enhancing their ductility and enabling low-temperature applications. Aluminum-gallium alloys offer improved corrosion resistance and electrical conductivity compared to pure aluminum.
Table of Comparison
| Property | Gallium | Aluminum |
|---|---|---|
| Atomic Number | 31 | 13 |
| Density (g/cm3) | 5.91 | 2.70 |
| Melting Point (degC) | 29.76 | 660.3 |
| Common Alloy Usage | Low, mainly semiconductor & specialized alloys | High, aerospace, automotive, construction |
| Corrosion Resistance | Moderate | High |
| Mechanical Strength | Low | Moderate to High |
| Thermal Conductivity (W/m*K) | 40 | 205 |
| Electrical Conductivity (MS/m) | 7.8 | 37.7 |
| Cost | Higher | Lower |
Introduction to Gallium and Aluminum Alloys
Gallium and aluminum alloys exhibit distinct properties due to their unique elemental characteristics, with gallium offering low melting points and excellent wetting abilities, while aluminum provides lightweight strength and corrosion resistance. Gallium alloys are often used in semiconductors and low-melting-point applications, whereas aluminum alloys dominate aerospace, automotive, and structural industries for their high strength-to-weight ratio. Understanding their metallurgical differences helps optimize alloy selection for specific industrial and technological applications.
Chemical and Physical Properties Comparison
Gallium exhibits a low melting point of about 29.76degC, enabling it to remain liquid near room temperature, while aluminum melts at 660.3degC, making it solid under normal conditions. Chemically, gallium forms stable gallium oxide with a thin oxide layer that self-heals rapidly, whereas aluminum forms a protective aluminum oxide layer that resists corrosion effectively. In alloys, gallium can weaken aluminum's crystal lattice and increase ductility, but also reduces overall strength and thermal conductivity compared to pure aluminum alloys.
Alloy Formation and Compatibility
Gallium significantly disrupts aluminum's crystal lattice during alloy formation, enhancing the material's malleability and lowering its melting point, which is beneficial for specialized applications like low-temperature soldering. The atomic radius and electronic configuration differences between gallium and aluminum lead to unique intermetallic compounds that influence mechanical strength and corrosion resistance. Compatibility concerns arise due to gallium's tendency to diffuse rapidly into aluminum, potentially causing embrittlement and structural weaknesses in conventional aluminum alloys.
Mechanical Strength and Durability
Gallium, when alloyed with aluminum, significantly improves mechanical strength by altering the alloy's microstructure, resulting in enhanced hardness and tensile strength compared to pure aluminum alloys. Aluminum-gallium alloys exhibit increased durability due to gallium's ability to inhibit grain growth and corrosion, extending the lifespan of components under harsh environmental conditions. These properties make aluminum-gallium alloys particularly suitable for aerospace and automotive applications requiring lightweight materials with robust mechanical performance.
Corrosion Resistance Analysis
Gallium exhibits superior corrosion resistance compared to aluminum when used in alloys, primarily due to its ability to form stable oxide layers that inhibit further oxidation. Aluminum alloys prone to pitting and galvanic corrosion show enhanced durability with gallium incorporation, reducing susceptibility to environmental degradation. Corrosion resistance tests reveal that gallium-alloyed metals maintain structural integrity longer in acidic and saline conditions, making gallium a valuable additive for enhancing alloy longevity.
Industrial Applications and Usages
Gallium enhances aluminum alloys by improving machinability and corrosion resistance, making them highly suitable for aerospace and electronics industries. Aluminum's lightweight and strong base properties combined with gallium create alloys that withstand extreme temperatures and mechanical stress in automotive and structural applications. Industrial usage of gallium-aluminum alloys is critical in semiconductor manufacturing, where gallium improves electrical conductivity and thermal stability.
Environmental Impact and Sustainability
Gallium alloys exhibit lower environmental impact compared to aluminum alloys due to their reduced energy-intensive extraction processes and greater potential for recycling. Aluminum production generates significant greenhouse gas emissions and requires substantial bauxite mining, which contributes to habitat destruction and soil erosion. Gallium's scarcity limits its large-scale use, but its incorporation in alloys can enhance sustainability by reducing overall material consumption and enabling longer-lasting, corrosion-resistant applications.
Cost and Economic Considerations
Gallium, though valued for enhancing aluminum alloys' corrosion resistance and strength, remains significantly more expensive than aluminum due to its rarity and limited production. Aluminum, abundant and cost-effective, dominates alloy usage in industries prioritizing economic efficiency, especially in automotive and aerospace sectors. The high cost of gallium restricts its widespread application, making aluminum alloys the preferred choice for large-scale manufacturing where budget constraints are critical.
Innovation and Future Developments
Gallium's low melting point and ability to create unique alloys with aluminum offer innovative pathways for developing lightweight, flexible materials in aerospace and electronics. Research is focused on enhancing the mechanical properties and corrosion resistance of gallium-aluminum alloys to surpass traditional aluminum-based alloys. Future developments include scalable manufacturing techniques and integration into smart materials for next-generation applications in energy storage and wearable technology.
Conclusion: Choosing the Right Alloy
Gallium alloys offer unique benefits such as low melting points and improved corrosion resistance, making them suitable for specialized applications like electronics and thermal management. Aluminum alloys excel in strength-to-weight ratio, cost-effectiveness, and widespread availability, ideal for structural and aerospace uses. Selecting the right alloy depends on balancing performance requirements with operational conditions, prioritizing gallium-based alloys for niche uses and aluminum for general-purpose, high-strength applications.
Infographic: Gallium vs Aluminum for Alloy
 azmater.com
azmater.com