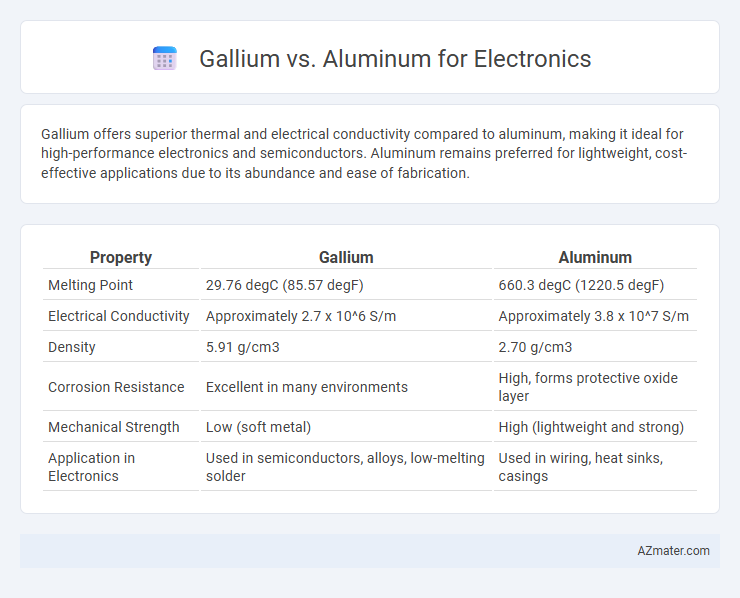
Gallium offers superior thermal and electrical conductivity compared to aluminum, making it ideal for high-performance electronics and semiconductors. Aluminum remains preferred for lightweight, cost-effective applications due to its abundance and ease of fabrication.
Table of Comparison
| Property | Gallium | Aluminum |
|---|---|---|
| Melting Point | 29.76 degC (85.57 degF) | 660.3 degC (1220.5 degF) |
| Electrical Conductivity | Approximately 2.7 x 10^6 S/m | Approximately 3.8 x 10^7 S/m |
| Density | 5.91 g/cm3 | 2.70 g/cm3 |
| Corrosion Resistance | Excellent in many environments | High, forms protective oxide layer |
| Mechanical Strength | Low (soft metal) | High (lightweight and strong) |
| Application in Electronics | Used in semiconductors, alloys, low-melting solder | Used in wiring, heat sinks, casings |
Introduction to Gallium and Aluminum in Electronics
Gallium and aluminum are essential metals in electronics, with aluminum widely used for its excellent electrical conductivity, lightweight properties, and cost-effectiveness in circuits and housings. Gallium, though less common, plays a critical role in semiconductor technology, particularly in gallium arsenide (GaAs) compounds that enable high-speed, high-frequency devices like RF components and LEDs. The distinct electronic and thermal characteristics of gallium-based materials complement aluminum's structural advantages, driving innovation in advanced electronic applications.
Material Properties: Gallium vs. Aluminum
Gallium exhibits a low melting point of about 29.76degC, enabling it to transition between solid and liquid states near room temperature, which contrasts with aluminum's melting point of 660.3degC, making aluminum more stable under typical electronic device operating conditions. Gallium demonstrates excellent wetting properties on glass and metals, enhancing its use in semiconductors and optoelectronics, while aluminum offers superior mechanical strength, electrical conductivity of approximately 37.7 million siemens per meter, and corrosion resistance due to its self-passivating oxide layer. The density difference, with gallium at 5.91 g/cm3 and aluminum at 2.70 g/cm3, impacts weight and heat dissipation in electronic components, influencing material selection based on device performance requirements.
Conductivity Comparison: Electrical and Thermal
Gallium exhibits superior thermal conductivity compared to aluminum, with approximately 40 W/(m*K) versus aluminum's 205 W/(m*K), making aluminum the better thermal conductor for heat dissipation in electronics. However, gallium's electrical conductivity, around 7.14 x 10^6 S/m, closely matches aluminum's 3.5 x 10^7 S/m, yet aluminum remains preferred due to lower cost and higher electrical conductivity. The unique properties of gallium, including its low melting point and liquid state near room temperature, offer niche applications despite aluminum's dominance in overall conductivity performance.
Melting Points and Temperature Stability
Gallium, with a melting point of approximately 29.76degC, is significantly lower than aluminum's melting point of about 660.32degC, making aluminum far more suitable for high-temperature electronics due to its superior thermal stability. Aluminum retains structural integrity and performance under elevated temperatures commonly found in electronic devices, while gallium's low melting point limits its use primarily to applications requiring low-temperature conductivity or phase change materials. The temperature stability of aluminum ensures reliable operation and longevity in electronic components compared to the more temperature-sensitive nature of gallium.
Reactivity and Corrosion Resistance
Gallium exhibits lower reactivity than aluminum, making it less prone to rapid oxidation in electronic components. Its superior corrosion resistance enhances durability, especially in humid or chemically aggressive environments, where aluminum tends to form protective oxide layers that can degrade over time. The choice of gallium over aluminum improves the longevity and performance of sensitive electronic devices by minimizing corrosion-related failures.
Manufacturing Processes and Scalability
Gallium's use in electronics manufacturing offers advantages due to its ability to form GaAs (gallium arsenide) semiconductors, which provide higher electron mobility and better performance at high frequencies compared to aluminum-based components. Aluminum, favored for its abundant availability and cost-effectiveness, is widely used in metallization layers and heat sinks, supporting scalable mass production through established processes like sputtering and vapor deposition. Scalability challenges with gallium arise from its higher cost and more complex crystal growth techniques, while aluminum benefits from mature, low-cost manufacturing infrastructure suitable for large-volume electronics production.
Performance in Semiconductor Applications
Gallium exhibits superior electron mobility and higher thermal conductivity compared to aluminum, making it ideal for high-frequency and high-power semiconductor devices. Gallium-based compounds, such as gallium arsenide (GaAs), enable faster switching speeds and greater efficiency in RF and microwave applications. Aluminum, while abundant and cost-effective, lacks the performance characteristics necessary for advanced semiconductor performance in cutting-edge electronics.
Cost Analysis: Gallium vs. Aluminum
Gallium's higher cost compared to aluminum significantly impacts electronics manufacturing budgets, with gallium priced at approximately $350 per kilogram while aluminum costs around $2 per kilogram. Despite aluminum's affordability, gallium's superior semiconductor properties justify its use in high-performance electronic components like LEDs and solar cells. Cost-effective aluminum remains dominant in general electronics casings and heat sinks, while gallium is reserved for specialized applications requiring enhanced conductivity and efficiency.
Environmental Impact and Sustainability
Gallium offers a lower environmental impact compared to aluminum due to its higher efficiency in electronics, which reduces energy consumption during device operation. While aluminum production is energy-intensive and generates significant greenhouse gas emissions, gallium is typically obtained as a byproduct of mining, minimizing additional environmental disturbance. Sustainable electronics benefit from gallium's potential for recycling and longer device lifespans, contributing to reduced resource depletion and waste in the tech industry.
Future Prospects in Electronics Industry
Gallium's superior semiconductor properties and higher electron mobility position it as a promising material for next-generation electronic devices, including high-speed transistors and optoelectronic components. Aluminum remains vital due to its low cost and excellent conductivity, primarily in interconnects and heat dissipation, but gallium-based compounds like gallium nitride (GaN) are rapidly advancing in power electronics and 5G technologies. Future prospects highlight gallium's potential to revolutionize electronics with enhanced efficiency and miniaturization, while aluminum continues to support mainstream production with cost-effective and reliable solutions.
Infographic: Gallium vs Aluminum for Electronics
 azmater.com
azmater.com