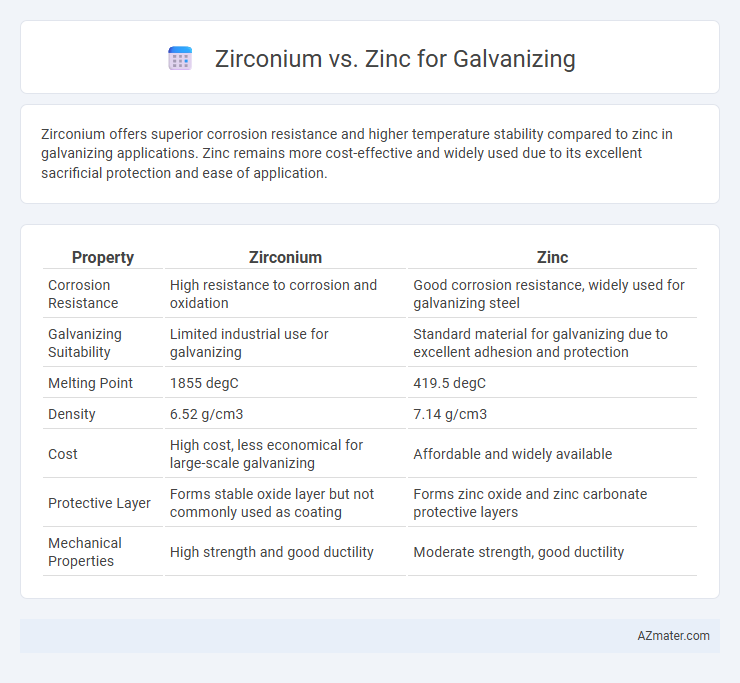
Zirconium offers superior corrosion resistance and higher temperature stability compared to zinc in galvanizing applications. Zinc remains more cost-effective and widely used due to its excellent sacrificial protection and ease of application.
Table of Comparison
| Property | Zirconium | Zinc |
|---|---|---|
| Corrosion Resistance | High resistance to corrosion and oxidation | Good corrosion resistance, widely used for galvanizing steel |
| Galvanizing Suitability | Limited industrial use for galvanizing | Standard material for galvanizing due to excellent adhesion and protection |
| Melting Point | 1855 degC | 419.5 degC |
| Density | 6.52 g/cm3 | 7.14 g/cm3 |
| Cost | High cost, less economical for large-scale galvanizing | Affordable and widely available |
| Protective Layer | Forms stable oxide layer but not commonly used as coating | Forms zinc oxide and zinc carbonate protective layers |
| Mechanical Properties | High strength and good ductility | Moderate strength, good ductility |
Introduction to Galvanizing: Purpose and Processes
Galvanizing is a protective coating process primarily used to prevent steel and iron corrosion by applying a layer of metal, commonly zinc, which acts as a sacrificial anode. Zinc is traditionally favored for galvanizing due to its excellent corrosion resistance, cost-effectiveness, and self-healing properties when scratched. Zirconium, although less common, offers superior resistance to high temperatures and abrasion, making it suitable for specialized galvanizing applications requiring enhanced durability.
Overview of Zirconium and Zinc in Metallurgy
Zirconium and zinc serve distinct roles in metallurgy, with zinc primarily used for galvanizing due to its excellent corrosion resistance and cost-effectiveness, providing a protective zinc coating to steel and iron. Zirconium, while less common in galvanizing, is valued for its high resistance to heat, corrosion, and wear, making it suitable for specialized alloying and protective coatings in extreme environments. Zinc's widespread application in galvanizing contrasts with zirconium's niche use in enhancing metallurgical properties where greater durability and chemical resistance are required.
Mechanisms of Corrosion Protection: Zirconium vs. Zinc
Zirconium provides corrosion protection primarily through the formation of a dense, stable oxide layer (ZrO2) that acts as a strong physical and chemical barrier against environmental attack. Zinc protects steel by serving as a sacrificial anode, corroding preferentially to the underlying metal and creating a protective patina of zinc carbonate that slows further corrosion. The key difference lies in zirconium's passive barrier mechanism enhancing resistance to aggressive environments, while zinc relies on active galvanic protection via sacrificial corrosion.
Environmental Impact and Sustainability
Zinc remains the dominant metal in galvanizing due to its proven durability and recyclability, with a strong global recycling rate exceeding 90%, minimizing environmental waste and resource depletion. Zirconium coatings, while less common and more costly, offer enhanced corrosion resistance and longer lifespan, potentially reducing the frequency of reapplication and associated environmental impacts over time. Sustainable galvanizing practices favor zinc for its established circular economy benefits, but ongoing research into zirconium's eco-friendly alloy potential could shift future sustainability paradigms.
Cost Comparison: Zirconium vs. Zinc Galvanizing
Zinc galvanizing remains the more cost-effective option compared to zirconium, with zinc prices typically ranging from $2,500 to $3,500 per ton, while zirconium costs can exceed $20,000 per ton due to its rarer availability and higher extraction expenses. Zinc's established supply chain and widespread use in galvanizing processes contribute to lower application and maintenance costs, making it the preferred choice for large-scale industrial projects. Though zirconium offers superior corrosion resistance, its significant cost premium limits its use to specialized applications where enhanced durability justifies the investment.
Performance and Durability in Harsh Conditions
Zirconium-based coatings offer superior corrosion resistance and enhanced durability compared to zinc in harsh environments, resisting oxidation and wear even under high temperatures and acidic exposure. Zinc galvanizing provides effective protection against rust by forming a sacrificial layer but tends to degrade faster than zirconium compounds when exposed to extreme moisture, salinity, or chemical stress. For long-term industrial applications requiring maximum performance in aggressive conditions, zirconium coatings deliver better maintenance intervals and extended service life.
Industrial Applications and Suitability
Zirconium offers superior corrosion resistance and high-temperature stability, making it ideal for specialized industrial applications such as aerospace and chemical processing. Zinc remains the preferred choice for galvanizing steel in construction and automotive industries due to its cost-effectiveness and excellent sacrificial protection against rust. While zinc is widely used for broad galvanizing purposes, zirconium's niche suitability lies in environments requiring enhanced durability and resistance to aggressive corrosion.
Health and Safety Considerations
Zirconium offers enhanced corrosion resistance and lower toxicity compared to zinc, making it a safer choice for galvanizing in environments with strict health regulations. Zinc galvanizing emits fumes containing harmful zinc oxide, which can cause respiratory issues such as metal fume fever if inhaled during welding or hot-dip processes. Proper ventilation and personal protective equipment are essential when handling zinc coatings, while zirconium's relatively inert properties reduce the risk of hazardous exposure.
Innovations and Future Trends in Galvanizing Materials
Zirconium-based coatings are emerging as innovative alternatives to traditional zinc in galvanizing due to their superior corrosion resistance and enhanced durability in harsh environments. Advances in nanotechnology have enabled the development of zirconium alloys with improved adhesion and environmental sustainability, addressing limitations of zinc such as susceptibility to white rust. Future trends indicate a growing adoption of zirconium-enhanced galvanizing materials driven by regulatory pressures and the need for longer-lasting protective coatings in automotive and construction industries.
Conclusion: Choosing Between Zirconium and Zinc
Zinc remains the preferred choice for galvanizing due to its proven corrosion resistance, cost-effectiveness, and widespread industry acceptance in steel protection. Zirconium coatings offer enhanced durability and high-temperature resistance but come at a higher price and limited application scope. Selecting between zirconium and zinc depends on specific project requirements, balancing budget constraints with desired performance characteristics.
Infographic: Zirconium vs Zinc for Galvanizing
 azmater.com
azmater.com