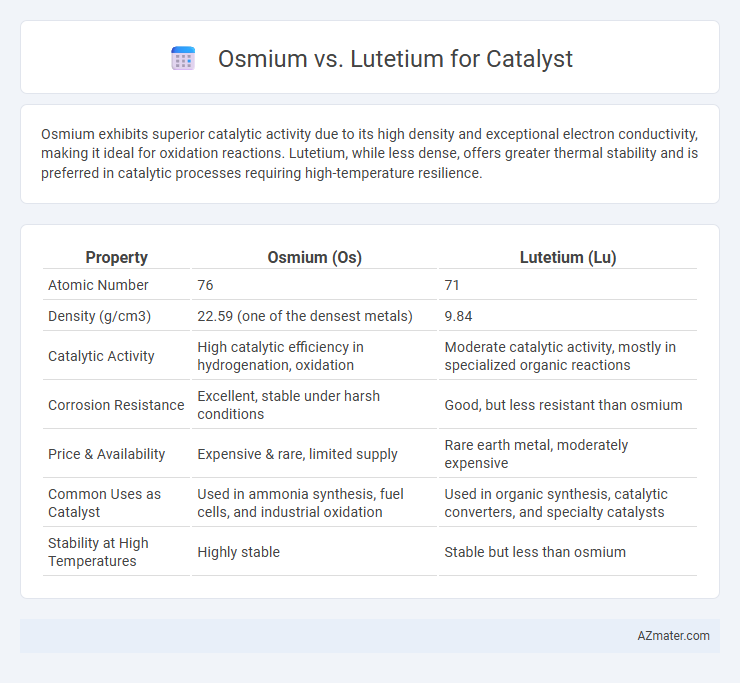
Osmium exhibits superior catalytic activity due to its high density and exceptional electron conductivity, making it ideal for oxidation reactions. Lutetium, while less dense, offers greater thermal stability and is preferred in catalytic processes requiring high-temperature resilience.
Table of Comparison
| Property | Osmium (Os) | Lutetium (Lu) |
|---|---|---|
| Atomic Number | 76 | 71 |
| Density (g/cm3) | 22.59 (one of the densest metals) | 9.84 |
| Catalytic Activity | High catalytic efficiency in hydrogenation, oxidation | Moderate catalytic activity, mostly in specialized organic reactions |
| Corrosion Resistance | Excellent, stable under harsh conditions | Good, but less resistant than osmium |
| Price & Availability | Expensive & rare, limited supply | Rare earth metal, moderately expensive |
| Common Uses as Catalyst | Used in ammonia synthesis, fuel cells, and industrial oxidation | Used in organic synthesis, catalytic converters, and specialty catalysts |
| Stability at High Temperatures | Highly stable | Stable but less than osmium |
Introduction to Osmium and Lutetium as Catalysts
Osmium and lutetium are both transition metals with distinct catalytic properties used in various chemical reactions. Osmium exhibits exceptional catalytic activity in oxidation and hydrogenation processes due to its high density and stability, making it ideal for industrial applications like fuel cells and organic synthesis. Lutetium, a rare earth metal with unique electronic configurations, serves as an effective catalyst in hydrocarbon cracking and polymerization reactions, offering advantages in selectivity and thermal stability.
Chemical Properties Relevant to Catalysis
Osmium exhibits exceptional catalytic activity due to its high density and ability to facilitate oxidation and hydrogenation reactions, making it valuable in various industrial processes. Lutetium, with its stable +3 oxidation state and robust Lewis acidity, shows promise in catalysis involving polymerization and organic transformations, though it is less explored compared to osmium. The electron-rich d-orbitals of osmium enable versatile coordination chemistry, whereas lutetium's smaller ionic radius and f-electron configuration influence its unique catalytic behavior in specific reactions.
Abundance and Availability
Osmium has a crustal abundance of approximately 0.001-0.01 ppm, making it one of the rarest platinum group metals with limited availability, primarily sourced from nickel and copper ores. Lutetium, although also a rare earth element with an abundance around 0.5 ppm in the Earth's crust, is more abundant than osmium and is mainly obtained from monazite and bastnasite minerals through complex extraction processes. The relative scarcity and challenging extraction of osmium limit its widespread catalytic applications, whereas lutetium's higher abundance and more accessible supply chains support its potential use in specialized catalytic roles.
Catalytic Mechanisms: Osmium vs Lutetium
Osmium exhibits high catalytic activity due to its ability to facilitate redox reactions through multiple oxidation states, making it ideal for hydrogenation and oxidation processes. Lutetium, as a lanthanide, primarily acts as a Lewis acid catalyst, stabilizing transition states in organic transformations such as polymerizations and cyclizations. The catalytic mechanism of osmium centers on electron transfer and surface adsorption, whereas lutetium relies on coordination chemistry and electrophilic activation, influencing their selectivity and reaction scope.
Applications in Industrial Catalysis
Osmium and lutetium both serve as catalysts with distinct industrial applications, where osmium excels in hydrogenation and oxidation reactions due to its high density and catalytic activity. Lutetium's catalytic role is prominent in polymerization processes and selective hydrogenation, benefiting from its stability in harsh chemical environments. Industries such as petrochemical refining and pharmaceutical synthesis leverage osmium for efficient catalytic conversions, while lutetium is favored for specialty chemical production and fine-tuning reaction selectivity.
Selectivity and Efficiency Comparison
Osmium catalysts exhibit superior selectivity in hydrogenation reactions due to their unique electronic structure and surface properties, enabling precise control over product formation. Lutetium-based catalysts offer high efficiency in specific organometallic transformations, driven by their strong Lewis acidity and stable coordination environment. Comparing both, osmium demonstrates enhanced selectivity for fine chemical synthesis, whereas lutetium excels in catalytic efficiency under milder conditions, impacting their practical application scope.
Environmental and Safety Considerations
Osmium catalysts often raise environmental concerns due to the toxicity of osmium tetroxide, a volatile compound posing significant health hazards and requiring strict handling protocols to prevent exposure and environmental contamination. Lutetium, being less toxic and more stable, presents a safer alternative with reduced risk for ecological harm and easier compliance with environmental regulations during catalytic processes. Selecting lutetium over osmium can mitigate hazardous waste generation and improve overall safety in industrial catalytic applications.
Economic Factors: Cost and Scalability
Osmium as a catalyst exhibits high catalytic activity but is limited by its extreme rarity and exorbitant cost, making it less economically viable for large-scale industrial applications. Lutetium, though also rare, offers relatively better cost-efficiency and more scalable availability due to its higher abundance in rare earth minerals and lower market price. Economically, lutetium's balance of performance and affordability supports more sustainable catalyst production and broader commercial deployment compared to osmium.
Recent Research and Innovations
Recent research highlights osmium's superior catalytic efficiency in hydrogenation and oxygen reduction reactions due to its robust electron density and stability under extreme conditions. Lutetium, while less studied, shows promise in selective catalytic processes involving hydrocarbon activation attributed to its unique 4f electron configuration. Innovations in nanoengineering techniques have enhanced osmium nanoparticle catalysts' surface area, significantly boosting their activity and durability in fuel cell applications.
Choosing the Right Catalyst: Osmium or Lutetium?
Osmium and Lutetium serve distinct roles in catalysis based on their electronic properties and reactivity; Osmium excels in hydrogenation and oxidation reactions due to its dense electron configuration and robustness. Lutetium, a rare earth metal, offers advantages in catalytic processes requiring strong Lewis acidity and high thermal stability, particularly in polymerization and organic synthesis. Selecting between Osmium and Lutetium catalysts depends on the specific reaction mechanism, operational conditions, and desired product yield, with Osmium favored for redox reactions and Lutetium for acid-catalyzed transformations.
Infographic: Osmium vs Lutetium for Catalyst
 azmater.com
azmater.com