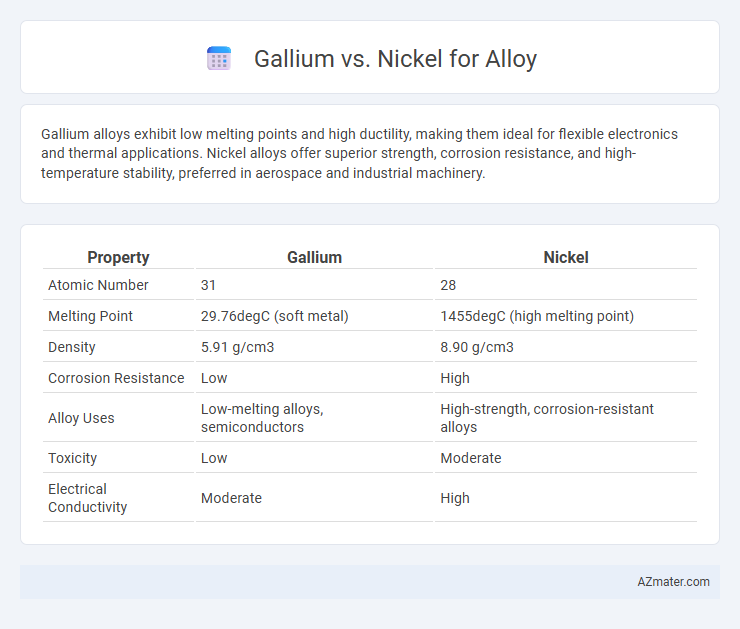
Gallium alloys exhibit low melting points and high ductility, making them ideal for flexible electronics and thermal applications. Nickel alloys offer superior strength, corrosion resistance, and high-temperature stability, preferred in aerospace and industrial machinery.
Table of Comparison
| Property | Gallium | Nickel |
|---|---|---|
| Atomic Number | 31 | 28 |
| Melting Point | 29.76degC (soft metal) | 1455degC (high melting point) |
| Density | 5.91 g/cm3 | 8.90 g/cm3 |
| Corrosion Resistance | Low | High |
| Alloy Uses | Low-melting alloys, semiconductors | High-strength, corrosion-resistant alloys |
| Toxicity | Low | Moderate |
| Electrical Conductivity | Moderate | High |
Introduction to Gallium and Nickel in Alloy Manufacturing
Gallium, a soft, silvery metal with a low melting point, is primarily used in alloy manufacturing to enhance properties such as ductility and corrosion resistance. Nickel, a hard, corrosion-resistant metal, is widely employed in alloys to improve strength, heat tolerance, and oxidation resistance. Both elements are critical in specialized alloy applications, with gallium often used in low-melting alloys and nickel dominating high-performance, high-temperature alloy systems.
Chemical and Physical Properties Comparison
Gallium exhibits a low melting point of 29.76degC and high malleability, making it ideal for alloys requiring fluidity and flexibility, while nickel features a high melting point of 1455degC, notable hardness, and excellent corrosion resistance, suitable for durable, high-strength alloys. Chemically, gallium is more reactive, forming stable compounds with oxygen and other nonmetals, whereas nickel's relatively inert nature contributes to its widespread use in corrosion-resistant and heat-resistant alloys. The distinct atomic structures and electron configurations of gallium (group 13, atomic number 31) and nickel (transition metal, atomic number 28) affect their alloying behavior, influencing properties such as conductivity, tensile strength, and resistance to oxidation.
Availability and Sourcing of Gallium vs Nickel
Gallium is significantly rarer than nickel, with global production concentrated mainly in a few countries like China, Germany, and Kazakhstan, making its sourcing more limited and potentially costly. Nickel is abundant and widely mined in countries such as Indonesia, the Philippines, Russia, and Canada, ensuring a more stable and diverse supply chain. The scarcity of gallium impacts its availability for alloy production, whereas nickel's extensive reserves support large-scale industrial use.
Melting Points and Temperature Resilience
Gallium has a low melting point of about 29.8degC, making it suitable for applications requiring low-temperature melting alloys, whereas nickel's melting point is significantly higher at 1455degC, offering superior temperature resilience. Nickel alloys maintain structural integrity and strength at elevated temperatures, making them ideal for high-temperature environments such as aerospace and industrial turbines. Gallium's limited temperature range confines its use primarily to low-melting alloys, while nickel's thermal stability supports high-performance, heat-resistant alloy applications.
Corrosion Resistance in Alloys
Gallium alloys typically exhibit enhanced corrosion resistance due to their ability to form stable, protective oxide layers, which effectively inhibit metal degradation in harsh environments. Nickel alloys are renowned for their superior corrosion resistance in acidic and high-temperature conditions, often used in chemical processing and marine applications due to their passive oxide films. While gallium improves wettability and flexibility in some alloys, nickel remains more reliable for long-term corrosion resistance in structurally demanding environments.
Mechanical Strength and Ductility Differences
Gallium alloys show superior ductility but lower mechanical strength compared to nickel-based alloys, which exhibit higher tensile strength and hardness. Gallium's low melting point and atomic structure allow for greater flexibility, making it suitable for applications requiring elasticity. Conversely, nickel alloys maintain structural integrity under high stress, offering enhanced durability in demanding mechanical environments.
Industrial Applications: Gallium-Based vs Nickel-Based Alloys
Gallium-based alloys exhibit exceptional low melting points and excellent wettability, making them ideal for industries requiring flexible thermal management and advanced electronic packaging. In contrast, nickel-based alloys offer superior strength, corrosion resistance, and high-temperature stability, which are critical for aerospace, power generation, and chemical processing applications. Industrial applications prioritize gallium alloys for thermal interface materials and nickel alloys for structural components subjected to extreme environments.
Environmental Impact and Recycling Considerations
Gallium alloys exhibit lower toxicity and reduced environmental footprint compared to nickel-based alloys, which often involve energy-intensive mining and refining processes with significant pollutant emissions. Gallium's recyclability benefits from its relatively low melting point and ability to be recovered with less environmental disturbance, while nickel's recycling is well-established but can generate hazardous waste requiring careful management. Prioritizing gallium alloys supports sustainable material cycles through lower lifecycle emissions and more efficient resource recovery in advanced alloy applications.
Cost Efficiency in Alloy Production
Gallium offers superior cost efficiency in alloy production due to its lower market price compared to nickel, reducing overall material expenses. Its ability to enhance mechanical properties at minimal concentrations leads to less raw material usage, further driving down production costs. Nickel's higher cost and relatively greater quantity requirements make it less economical for large-scale alloy manufacturing.
Future Prospects and Technological Innovations
Gallium alloys exhibit promising future prospects due to their low melting point and superior corrosion resistance, making them ideal for flexible electronics and next-generation semiconductors. Nickel alloys maintain technological importance with advances in high-temperature strength and magnetic properties, essential for aerospace and energy applications. Innovations in gallium-nickel composite materials demonstrate potential for enhanced conductivity and mechanical performance in emerging nanotechnology and energy storage devices.
Infographic: Gallium vs Nickel for Alloy
 azmater.com
azmater.com