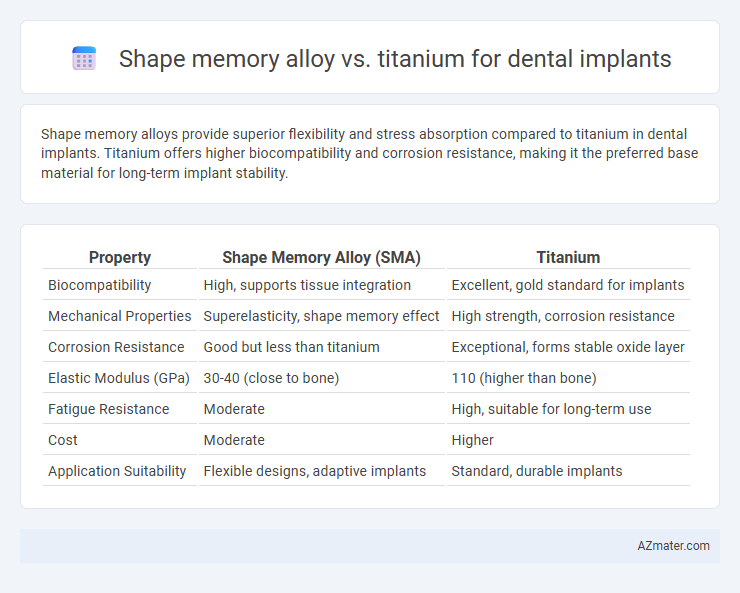
Shape memory alloys provide superior flexibility and stress absorption compared to titanium in dental implants. Titanium offers higher biocompatibility and corrosion resistance, making it the preferred base material for long-term implant stability.
Table of Comparison
| Property | Shape Memory Alloy (SMA) | Titanium |
|---|---|---|
| Biocompatibility | High, supports tissue integration | Excellent, gold standard for implants |
| Mechanical Properties | Superelasticity, shape memory effect | High strength, corrosion resistance |
| Corrosion Resistance | Good but less than titanium | Exceptional, forms stable oxide layer |
| Elastic Modulus (GPa) | 30-40 (close to bone) | 110 (higher than bone) |
| Fatigue Resistance | Moderate | High, suitable for long-term use |
| Cost | Moderate | Higher |
| Application Suitability | Flexible designs, adaptive implants | Standard, durable implants |
Introduction to Dental Implant Materials
Dental implant materials must exhibit biocompatibility, strength, and corrosion resistance to ensure long-term success. Shape memory alloys, such as nickel-titanium, offer unique elasticity and the ability to regain original shape after deformation, enhancing implant adaptability in dynamic oral environments. Titanium remains the gold standard for dental implants due to its excellent osseointegration, mechanical strength, and proven clinical performance.
Overview of Shape Memory Alloys in Dentistry
Shape memory alloys (SMAs), particularly nickel-titanium (NiTi), exhibit unique properties such as superelasticity and shape memory effect, making them highly suitable for dental implants and orthodontic applications. Their ability to withstand mechanical stress and return to their original shape enhances implant stability and patient comfort. Compared to titanium, SMAs offer improved flexibility and fatigue resistance, which can lead to longer-lasting and more adaptable dental prosthetics.
Titanium: The Gold Standard for Dental Implants
Titanium remains the gold standard for dental implants due to its exceptional biocompatibility, osseointegration capabilities, and corrosion resistance, which ensure long-term stability and success in oral environments. Its mechanical strength and ability to integrate seamlessly with bone tissue reduce implant failure rates compared to shape memory alloys, which may lack comparable biological compatibility and durability. Clinical studies consistently demonstrate titanium's superior performance in supporting dental prosthetics, making it the preferred choice for implant dentistry.
Biocompatibility: Shape Memory Alloy vs Titanium
Shape memory alloys, commonly nickel-titanium (NiTi), exhibit excellent biocompatibility due to their corrosion resistance and ability to integrate with surrounding tissues, but nickel content may pose allergenic risks for sensitive patients. Titanium is widely regarded as the gold standard for dental implants, offering superior osseointegration, outstanding corrosion resistance, and minimal immunological response, making it highly biocompatible in the oral environment. While both materials support bone regeneration, titanium's long-term clinical success and lower cytotoxicity make it the preferred choice for dental implant biocompatibility.
Mechanical Properties and Performance Comparison
Shape memory alloys, such as NiTi, exhibit superelasticity and excellent fatigue resistance, allowing dental implants to endure cyclic masticatory forces without permanent deformation. Titanium offers superior biocompatibility, high tensile strength, and corrosion resistance, making it the gold standard for implant osseointegration and long-term stability. Performance comparisons highlight titanium's consistent mechanical integrity and corrosion resistance, while shape memory alloys provide adaptive elasticity but face challenges with biocompatibility and potential nickel ion release.
Osseointegration Capabilities
Shape memory alloys (SMAs) exhibit excellent adaptability due to their superelasticity and biocompatibility, promoting enhanced early-stage osseointegration by minimizing micromovements at the implant-bone interface. Titanium remains the gold standard in dental implants owing to its superior corrosion resistance and well-documented capacity to form strong, stable oxide layers that facilitate long-term osseointegration through direct bone bonding. Comparative studies indicate titanium's consistent performance in achieving higher bone-to-implant contact ratios, whereas SMAs offer promising potential in dynamic stress environments but require further clinical validation.
Corrosion Resistance and Longevity
Shape memory alloys, such as NiTi, exhibit excellent corrosion resistance due to the formation of a stable oxide layer that protects the implant in the oral environment, whereas Titanium forms a robust titanium oxide film that offers superior biocompatibility and corrosion resistance. Titanium implants have demonstrated longer clinical longevity, often exceeding 15 years, attributed to their strong resistance to corrosion and mechanical stability under cyclic loading. While shape memory alloys provide unique flexibility and stress distribution benefits, Titanium remains the gold standard for dental implants due to its proven durability and exceptional corrosion resistance in saliva and biofilm exposure.
Clinical Applications and Case Studies
Shape memory alloys (SMAs), particularly nickel-titanium (NiTi), offer superior flexibility and stress distribution in dental implants, enhancing osseointegration and patient comfort in clinical applications. Titanium remains the gold standard due to its exceptional biocompatibility, corrosion resistance, and long-term stability, supported by extensive case studies demonstrating high success rates in various prosthetic rehabilitations. Comparative studies reveal that SMAs provide improved adaptability in managing complex implant cases involving dynamic loading, while titanium implants consistently deliver predictable outcomes in bone integration and durability.
Cost-Effectiveness and Accessibility
Shape memory alloys (SMAs) like NiTi offer cost-effective dental implant options due to lower material and manufacturing expenses compared with titanium, making them accessible in markets with budget constraints. Titanium implants, although more expensive, provide superior biocompatibility and long-term durability, resulting in fewer replacement costs over time. The choice between SMA and titanium impacts overall investment, where SMAs prioritize initial affordability and titanium ensures enhanced longevity and clinical success in dental restorations.
Future Trends and Innovations in Dental Implant Materials
Shape memory alloys (SMAs) and titanium both exhibit promising future trends in dental implant materials, with SMAs offering enhanced adaptability through their superelastic and shape memory properties that promote better osseointegration and stress distribution. Innovations focus on surface modifications for titanium implants, such as nanoscale coatings and bioactive treatments to improve biocompatibility and accelerate healing. Emerging research on combining titanium's strength with SMA's flexibility aims to develop hybrid implants that optimize durability, patient comfort, and long-term performance.
Infographic: Shape memory alloy vs Titanium for Dental implant
 azmater.com
azmater.com