Iridium offers exceptional corrosion resistance and stability in battery catalysts, enhancing durability in fuel cells, while lithium provides high energy density and lightweight properties crucial for rechargeable batteries. Combining iridium's catalytic efficiency with lithium's energy storage capacity advances next-generation battery performance.
Table of Comparison
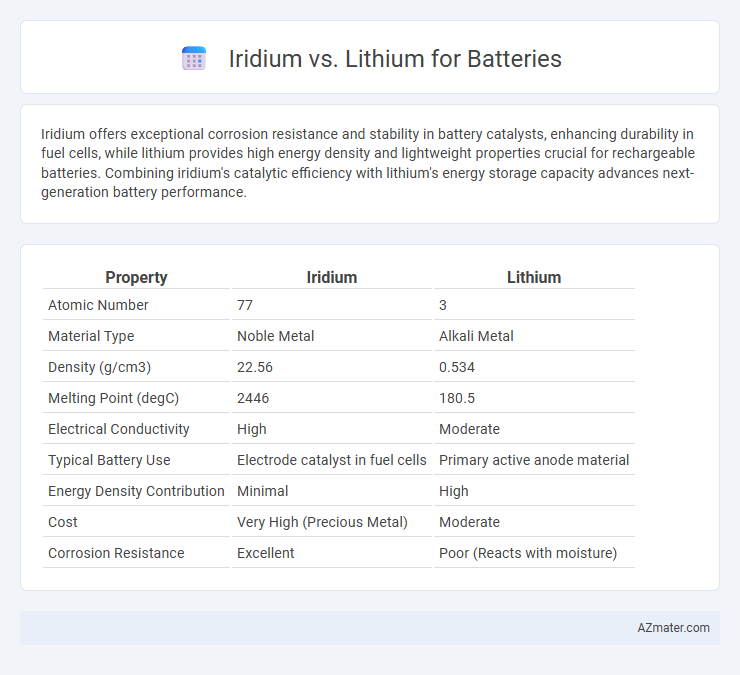
| Property | Iridium | Lithium |
|---|---|---|
| Atomic Number | 77 | 3 |
| Material Type | Noble Metal | Alkali Metal |
| Density (g/cm3) | 22.56 | 0.534 |
| Melting Point (degC) | 2446 | 180.5 |
| Electrical Conductivity | High | Moderate |
| Typical Battery Use | Electrode catalyst in fuel cells | Primary active anode material |
| Energy Density Contribution | Minimal | High |
| Cost | Very High (Precious Metal) | Moderate |
| Corrosion Resistance | Excellent | Poor (Reacts with moisture) |
Introduction to Iridium and Lithium in Battery Technology
Iridium, a rare transition metal, is primarily used in battery technology as a catalyst in fuel cells to enhance oxygen reduction reactions, improving efficiency and durability. Lithium, a lightweight alkali metal, forms the core of most modern rechargeable batteries due to its high energy density, enabling longer-lasting and faster-charging devices. Both elements play critical roles in advancing battery performance, with iridium focusing on catalytic support and lithium serving as the essential charge carrier.
Chemical Properties: Iridium vs Lithium
Iridium exhibits high corrosion resistance and excellent catalytic properties due to its dense electron configuration and stable oxidation states, making it valuable in specialized battery applications like electrodes and catalysts. Lithium's lightweight, high electrochemical potential, and low atomic mass contribute to its superior energy density and fast ion mobility, which are crucial in rechargeable lithium-ion batteries. The chemical stability of iridium contrasts sharply with lithium's high reactivity and tendency to form alloys, impacting their respective roles in battery efficiency and safety.
Energy Density Comparison
Iridium and lithium differ significantly in energy density, with lithium-based batteries typically offering much higher energy storage per unit weight and volume, making them ideal for portable electronics and electric vehicles. Iridium, used mainly in specialized applications such as catalysts in fuel cells or as electrode materials, possesses lower intrinsic energy density compared to lithium but contributes to battery durability and high-temperature performance. Advances in lithium-ion technology continue to push energy density limits beyond 300 Wh/kg, whereas iridium-related materials often enhance battery stability rather than raw energy capacity.
Cycle Life and Durability
Iridium-based batteries offer superior cycle life and durability due to the metal's exceptional corrosion resistance and stability under high-stress conditions. Lithium batteries, while widely used and efficient, often experience capacity degradation after several hundred to a few thousand charge cycles, limiting their overall lifespan. The enhanced stability of iridium electrodes can significantly extend battery longevity, making them ideal for applications requiring long-term reliability.
Cost and Material Availability
Iridium is significantly more expensive than lithium due to its rarity and complex extraction process, making it less viable for large-scale battery production. Lithium, abundant in countries like Australia, Chile, and Argentina, offers a cost-effective and widely available material base essential for mass manufacturing of lithium-ion batteries. The high cost and limited availability of iridium constrain its use to specialized battery applications, whereas lithium's accessibility supports the growing global demand in electric vehicles and energy storage systems.
Environmental Impact and Sustainability
Iridium is primarily used as a catalyst in certain battery technologies and is scarce, making its environmental footprint significant due to intensive mining and limited recycling options. Lithium, widely used in rechargeable batteries for electric vehicles and electronics, involves extensive mining processes that pose challenges such as water depletion and habitat disruption, but advancements in sustainable sourcing and recycling are mitigating these impacts. Sustainable battery development increasingly emphasizes reducing reliance on rare metals like iridium while improving lithium extraction methods to balance environmental concerns with growing energy storage demands.
Safety Profiles: Iridium-Based vs Lithium-Based Batteries
Iridium-based batteries exhibit enhanced safety profiles due to their higher thermal stability and resistance to overheating compared to lithium-based batteries, which are prone to thermal runaway and fire hazards. The robust chemical structure of iridium compounds minimizes the risk of electrolyte decomposition and dendrite formation, reducing the likelihood of short circuits. Lithium batteries require complex safety management systems, whereas iridium-based cells inherently offer improved operational safety in high-demand applications.
Current and Emerging Applications
Iridium-based catalysts exhibit high durability and efficiency in fuel cells and electrolyzers, making them suitable for current applications in hydrogen production and energy storage systems where long cycle life is critical. Lithium-ion batteries dominate emerging electric vehicle and grid storage markets due to their high energy density, rapid charge capabilities, and decreasing manufacturing costs, driving advancements in portable electronics and renewable energy integration. While iridium supports electrochemical reactions in specialized energy conversion technologies, lithium technology remains central to scalable, rechargeable battery solutions across multiple industries.
Technological Challenges and Future Prospects
Iridium batteries face significant technological challenges such as high material costs and limited electrode efficiency, which hinder large-scale commercial deployment compared to lithium-ion batteries that benefit from established manufacturing processes and higher energy densities. Lithium batteries still struggle with safety issues, including thermal runaway and limited lifespan, prompting research into solid-state electrolytes and silicon anodes to enhance performance and durability. Future prospects for iridium-based batteries hinge on breakthroughs in catalyst design and recycling methods, while lithium technology advances focus on improving energy density, charge rates, and sustainability through next-generation materials and battery management systems.
Conclusion: Which Element is Better for Batteries?
Lithium remains the superior element for batteries due to its high energy density, light weight, and widespread availability, making it ideal for applications from smartphones to electric vehicles. Iridium, while offering excellent catalytic properties and stability in specialized battery components like electrodes, is limited by its rarity and high cost. Therefore, lithium dominates battery technology, balancing performance and affordability for large-scale energy storage.
Infographic: Iridium vs Lithium for Battery
 azmater.com
azmater.com