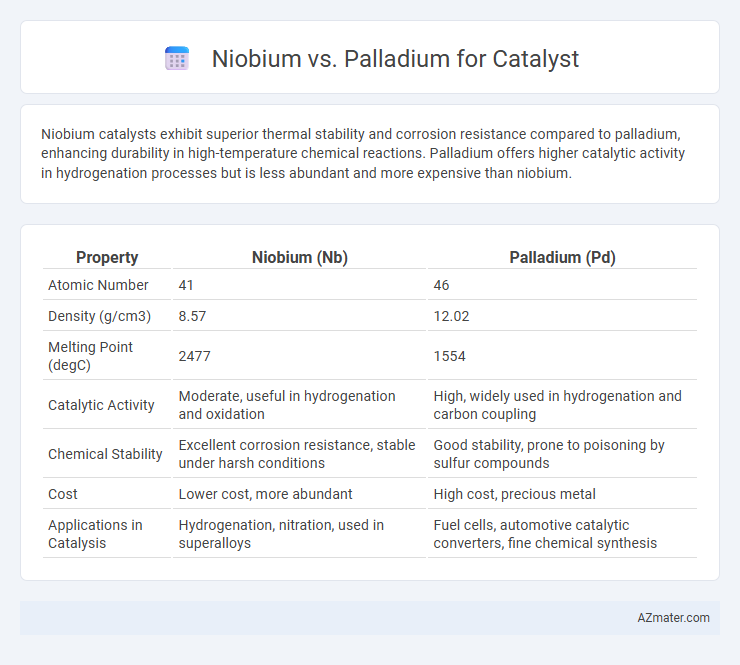
Niobium catalysts exhibit superior thermal stability and corrosion resistance compared to palladium, enhancing durability in high-temperature chemical reactions. Palladium offers higher catalytic activity in hydrogenation processes but is less abundant and more expensive than niobium.
Table of Comparison
| Property | Niobium (Nb) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 41 | 46 |
| Density (g/cm3) | 8.57 | 12.02 |
| Melting Point (degC) | 2477 | 1554 |
| Catalytic Activity | Moderate, useful in hydrogenation and oxidation | High, widely used in hydrogenation and carbon coupling |
| Chemical Stability | Excellent corrosion resistance, stable under harsh conditions | Good stability, prone to poisoning by sulfur compounds |
| Cost | Lower cost, more abundant | High cost, precious metal |
| Applications in Catalysis | Hydrogenation, nitration, used in superalloys | Fuel cells, automotive catalytic converters, fine chemical synthesis |
Introduction to Niobium and Palladium as Catalysts
Niobium and palladium serve crucial roles as catalysts in chemical reactions, with palladium extensively utilized for its superior ability to facilitate hydrogenation and carbon-carbon coupling processes. Niobium, though less common, exhibits promising catalytic properties in oxidation and acid-catalyzed reactions, benefiting from its high thermal stability and resistance to corrosion. Both metals contribute unique advantages in catalysis, driving advancements in fuel cells, automotive exhaust treatment, and fine chemical synthesis.
Chemical Properties Relevant to Catalysis
Niobium exhibits high thermal stability and strong acid-base properties, making it effective in catalytic reactions involving acid sites and oxidation processes. Palladium is renowned for its excellent hydrogen absorption capacity and ability to facilitate hydrogenation and carbon-carbon coupling reactions due to its electronic configuration. The differing chemical properties of niobium and palladium influence their catalytic selectivity, with niobium favoring acid-catalyzed mechanisms and palladium excelling in redox and hydrogenation reactions.
Catalytic Efficiency: Niobium vs Palladium
Niobium exhibits high catalytic efficiency in oxidation reactions due to its stable surface oxides and strong oxygen affinity, which enhance electron transfer processes. Palladium outperforms niobium in hydrogenation and carbon-carbon coupling reactions, offering superior activity and selectivity thanks to its unique d-band electronic structure. When comparing catalytic efficiency, palladium typically provides faster reaction rates and lower activation energies in hydrogen-involved processes, while niobium excels in oxidation catalysis under harsh conditions.
Selectivity and Reaction Specificity
Niobium catalysts exhibit superior selectivity in oxidation reactions due to their strong acidic sites and unique electronic properties, enhancing reaction specificity in partial oxidation processes. Palladium catalysts excel in hydrogenation and coupling reactions, offering high selectivity by preferentially activating specific bonds like C-C and C-H under mild conditions. The choice between niobium and palladium hinges on the targeted reaction pathway, with niobium favoring acid-catalyzed transformations and palladium enabling precise metal-catalyzed bond activation.
Stability and Resistance to Deactivation
Niobium catalysts exhibit superior thermal stability and resistance to deactivation compared to palladium, making them ideal for high-temperature industrial processes. Niobium's strong metal-support interaction minimizes sintering and coke formation, preserving catalytic activity over extended periods. Palladium, while highly active, is more prone to poisoning and sintering, leading to faster decline in performance under harsh reaction conditions.
Cost and Economic Considerations
Niobium-based catalysts are generally more cost-effective than palladium due to the lower market price and higher natural abundance of niobium, which reduces raw material expenses significantly. Palladium, a precious metal with volatile pricing, incurs higher costs in catalyst production and recycling processes, impacting overall economic feasibility in large-scale applications. The economic advantage of niobium is enhanced by its durability and resistance to poisoning, potentially lowering replacement frequency and operational costs compared to palladium catalysts.
Environmental Impact and Sustainability
Niobium catalysts demonstrate lower environmental impact due to their abundance, non-toxic nature, and high recyclability compared to palladium catalysts, which are scarce, expensive, and often involve mining processes with significant ecological footprints. Niobium-based catalysts contribute to sustainability by enabling energy-efficient reactions and reducing reliance on precious metals whose extraction leads to habitat destruction and pollution. The shift toward niobium enhances green chemistry efforts by minimizing hazardous waste and promoting circular economy principles in catalytic applications.
Applications in Industrial Catalysis
Niobium exhibits high thermal stability and strong acid-base properties, making it ideal for catalytic cracking and dehydration reactions in petrochemical industries. Palladium is widely used for hydrogenation, carbon-carbon coupling, and automotive exhaust treatment due to its exceptional hydrogen absorption and catalytic selectivity. Industrial catalysis applications leverage niobium for solid acid catalysis and palladium for fine chemical synthesis and emission control catalysts.
Recent Research and Innovations
Recent research highlights niobium's rising prominence in catalyst applications due to its exceptional thermal stability and resistance to poisoning, outperforming palladium in high-temperature reactions. Innovations in niobium-based catalysts have demonstrated significant improvements in hydrogenation and oxidation processes, driven by enhanced electronic properties and surface area optimization. While palladium remains effective for selective catalytic conversions, niobium's cost-efficiency and durability are accelerating its integration into sustainable catalytic systems.
Future Perspectives and Emerging Trends
Niobium offers promising prospects as a catalyst due to its exceptional thermal stability and resistance to deactivation, making it suitable for sustainable energy and environmental applications. Emerging trends highlight the integration of niobium with nanostructured supports to enhance catalytic efficiency and selectivity in hydrogen evolution and carbon dioxide reduction reactions. Palladium remains vital for catalytic converters and fuel cells, but future research is increasingly exploring niobium-based catalysts as cost-effective, earth-abundant alternatives with comparable performance.
Infographic: Niobium vs Palladium for Catalyst
 azmater.com
azmater.com