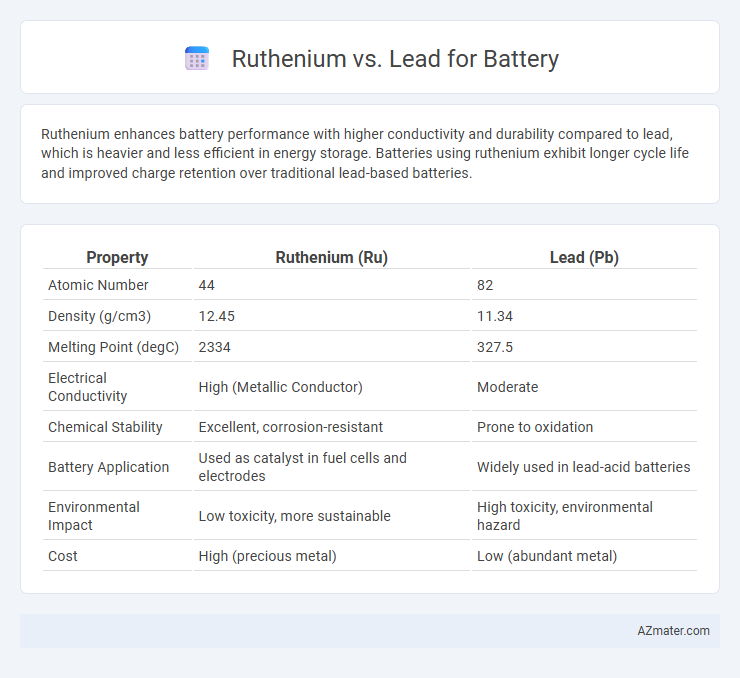
Ruthenium enhances battery performance with higher conductivity and durability compared to lead, which is heavier and less efficient in energy storage. Batteries using ruthenium exhibit longer cycle life and improved charge retention over traditional lead-based batteries.
Table of Comparison
| Property | Ruthenium (Ru) | Lead (Pb) |
|---|---|---|
| Atomic Number | 44 | 82 |
| Density (g/cm3) | 12.45 | 11.34 |
| Melting Point (degC) | 2334 | 327.5 |
| Electrical Conductivity | High (Metallic Conductor) | Moderate |
| Chemical Stability | Excellent, corrosion-resistant | Prone to oxidation |
| Battery Application | Used as catalyst in fuel cells and electrodes | Widely used in lead-acid batteries |
| Environmental Impact | Low toxicity, more sustainable | High toxicity, environmental hazard |
| Cost | High (precious metal) | Low (abundant metal) |
Introduction to Ruthenium and Lead in Battery Technology
Ruthenium in battery technology enhances electrode stability and improves cycle life due to its excellent catalytic properties and corrosion resistance, making it valuable in advanced lithium-ion and flow batteries. Lead, traditionally used in lead-acid batteries, offers cost-effectiveness and reliability but suffers from lower energy density and slower charge rates compared to newer materials. The integration of ruthenium aims to overcome limitations of lead-based systems by boosting efficiency and lifespan in rechargeable batteries.
Chemical Properties of Ruthenium and Lead
Ruthenium exhibits high corrosion resistance and excellent catalytic properties due to its stable oxidation states ranging from +2 to +8, making it valuable in enhancing battery electrode performance. Lead, commonly used in lead-acid batteries, features a +2 oxidation state and is characterized by moderate toxicity and relatively low corrosion resistance compared to ruthenium. The chemical stability and conductivity of ruthenium contribute to improved battery efficiency, while lead's heavier atomic mass and limited electrochemical versatility affect energy density and cycling stability.
Battery Performance: Efficiency and Capacity Comparison
Ruthenium-based batteries exhibit higher efficiency and longer cycle life compared to lead-acid batteries, enabling greater energy density and improved charge retention. Ruthenium's superior conductivity and catalytic properties enhance charge-discharge rates, translating into faster battery response times and higher capacity utilization. In contrast, lead-acid batteries have lower energy density and suffer from sulfation, which reduces their efficiency and overall capacity over extended use.
Electrochemical Stability of Ruthenium vs Lead
Ruthenium exhibits superior electrochemical stability compared to lead due to its higher resistance to corrosion and lower susceptibility to sulfation in battery applications. This stability enhances cycle life and charge retention, making ruthenium-based electrodes more efficient in maintaining consistent performance under prolonged charge-discharge conditions. Lead, while cost-effective, often suffers from lead sulfate formation, which degrades battery capacity and shortens service life.
Environmental Impact and Sustainability
Ruthenium-based batteries exhibit lower toxicity levels and higher recyclability compared to lead-acid batteries, reducing environmental contamination risks associated with heavy metal leakage. Lead batteries pose significant ecological hazards due to lead's toxic nature, which can cause soil and water pollution, making disposal and recycling challenging. Ruthenium's superior sustainability profile stems from its longer lifecycle and reduced hazardous waste generation, supporting greener energy storage solutions.
Cost Analysis: Ruthenium vs Lead Batteries
Ruthenium batteries exhibit higher initial material costs compared to lead batteries due to the rarity and expense of ruthenium metal, impacting overall production expenses. Lead batteries benefit from well-established manufacturing processes and abundant raw materials, resulting in lower cost per kilowatt-hour and widespread affordability. However, ruthenium batteries may offer longer lifespan and higher energy density, potentially offsetting upfront costs through improved performance and reduced replacement frequency.
Safety Considerations in Battery Applications
Ruthenium offers superior thermal stability and resistance to dendrite formation compared to lead, enhancing overall battery safety by reducing the risk of short circuits and thermal runaway. Lead-based batteries, while cost-effective, pose higher risks of acid leaks and hazardous heavy metal exposure, which can lead to both environmental and health safety concerns. Utilizing ruthenium in battery electrodes improves cycle life and safety margins, making it a safer alternative in advanced energy storage systems.
Lifespan and Durability of Ruthenium and Lead-Based Batteries
Ruthenium-based batteries exhibit significantly enhanced lifespan and durability compared to traditional lead-acid batteries due to ruthenium's superior corrosion resistance and stable electrochemical properties. The incorporation of ruthenium oxides in battery electrodes minimizes degradation processes, enabling longer cycle life and retention of capacity over time. Lead-based batteries, while cost-effective, suffer from sulfation and grid corrosion, resulting in shorter operational life and reduced reliability under high-demand conditions.
Current and Emerging Applications
Ruthenium-based compounds excel in battery applications due to their excellent catalytic properties and high electrical conductivity, enhancing the performance of lithium-ion and flow batteries. Lead remains a dominant material for traditional lead-acid batteries, widely used in automotive starter batteries and backup power systems, owing to its low cost and recyclability. Emerging applications explore ruthenium's role in advanced energy storage systems like supercapacitors and lithium-sulfur batteries, potentially surpassing lead in energy density and cycle life.
Future Prospects and Research Directions
Ruthenium-based batteries demonstrate promising future prospects due to their high catalytic activity and superior cycle stability, making them ideal for advanced energy storage applications. Research increasingly focuses on enhancing ruthenium's cost-effectiveness and scalability through nano-structuring and alloy development to outperform traditional lead-acid batteries. Innovations in ruthenium electrode materials aim to address lead battery limitations like low energy density and poor environmental sustainability, positioning ruthenium as a potential leader in next-generation battery technologies.
Infographic: Ruthenium vs Lead for Battery
 azmater.com
azmater.com