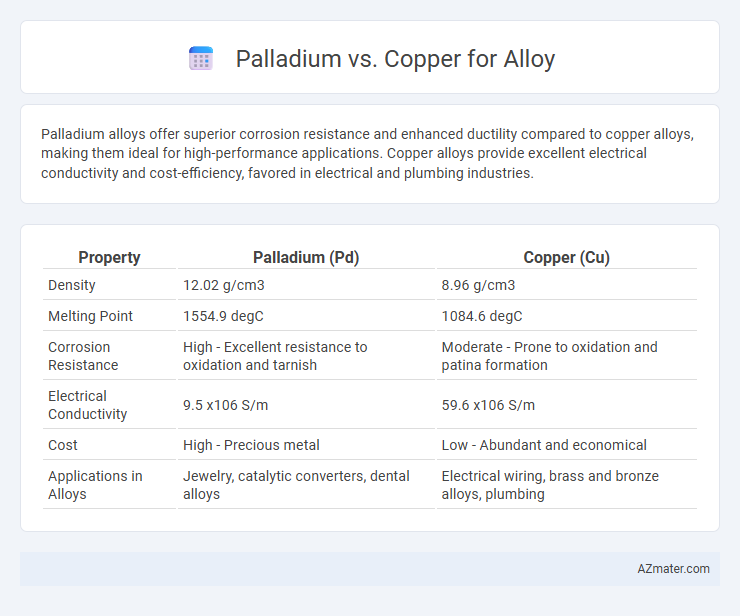
Palladium alloys offer superior corrosion resistance and enhanced ductility compared to copper alloys, making them ideal for high-performance applications. Copper alloys provide excellent electrical conductivity and cost-efficiency, favored in electrical and plumbing industries.
Table of Comparison
| Property | Palladium (Pd) | Copper (Cu) |
|---|---|---|
| Density | 12.02 g/cm3 | 8.96 g/cm3 |
| Melting Point | 1554.9 degC | 1084.6 degC |
| Corrosion Resistance | High - Excellent resistance to oxidation and tarnish | Moderate - Prone to oxidation and patina formation |
| Electrical Conductivity | 9.5 x106 S/m | 59.6 x106 S/m |
| Cost | High - Precious metal | Low - Abundant and economical |
| Applications in Alloys | Jewelry, catalytic converters, dental alloys | Electrical wiring, brass and bronze alloys, plumbing |
Introduction to Palladium and Copper in Alloys
Palladium, a rare precious metal, is highly valued in alloy production for its excellent corrosion resistance, catalytic properties, and ability to enhance strength and whiteness in precious metal alloys. Copper, a widely used base metal, offers superior electrical conductivity, ductility, and antimicrobial properties, making it a fundamental component in various industrial and decorative alloys. Combining palladium with copper in alloys often results in materials with improved durability, malleability, and resistance to tarnish, essential for applications in electronics, jewelry, and automotive industries.
Chemical and Physical Properties Overview
Palladium exhibits excellent corrosion resistance, high melting point around 1555degC, and superior catalytic properties, making it ideal for specialized alloy applications. Copper offers high electrical and thermal conductivity with a melting point of about 1085degC, combined with good ductility and antimicrobial characteristics in alloys. The choice between palladium and copper alloys depends largely on the desired chemical stability, conductivity, and mechanical strength requirements.
Alloying Capabilities and Compatibility
Palladium offers superior alloying capabilities with precious metals such as gold and platinum, enhancing corrosion resistance and strength without compromising ductility. Copper excels in improving hardness and thermal conductivity in alloys but may increase susceptibility to oxidation and tarnishing. Compatibility-wise, palladium forms stable, homogeneous alloys ideal for fine jewelry and electronics, while copper alloys are preferred in industrial applications requiring enhanced mechanical durability and electrical conductivity.
Mechanical Strength and Durability Comparison
Palladium-copper alloys exhibit superior mechanical strength compared to pure copper, with palladium enhancing hardness and tensile strength while maintaining excellent corrosion resistance. These alloys demonstrate increased durability in harsh environments due to palladium's ability to prevent oxidation and improve wear resistance, making them ideal for high-performance applications. Copper alone offers good conductivity and moderate strength but tends to suffer from faster degradation and lower mechanical resilience under stress.
Corrosion Resistance: Palladium vs Copper
Palladium exhibits superior corrosion resistance compared to copper, making it a preferred choice for alloys used in harsh environments and electronic components. Copper alloys tend to oxidize and develop patina over time, which can compromise structural integrity and conductivity. The inert nature of palladium prevents tarnish and degradation, enhancing the longevity and durability of palladium-containing alloys in marine, chemical, and automotive applications.
Cost and Economic Considerations
Palladium, significantly more expensive than copper, drives up alloy production costs, making it less economical for large-scale industrial applications. Copper's affordability and wide availability offer substantial cost savings, especially in sectors where budget constraints are critical. Economic considerations often favor copper alloys due to their balance of performance and lower material expenses compared to palladium-based alloys.
Applications in Industry and Technology
Palladium-copper alloys are widely used in the electronics industry for their excellent conductivity and corrosion resistance, making them ideal for connectors, switches, and microelectronic components. In automotive and aerospace sectors, these alloys offer enhanced durability and thermal stability, supporting applications in fuel cells and catalytic converters. The combination of palladium's noble metal properties with copper's affordability also benefits precision instruments and jewelry manufacturing, providing a balance between performance and cost.
Conductivity: Electrical and Thermal Performance
Palladium exhibits lower electrical conductivity compared to copper, with copper boasting one of the highest electrical conductivities among metals, making it ideal for efficient electrical transmission. In terms of thermal conductivity, copper outperforms palladium significantly, facilitating superior heat dissipation in applications requiring effective thermal management. Copper's combination of high electrical and thermal conductivity makes it a preferred choice for alloys in electronics and heat-sensitive environments, whereas palladium alloys are selected for corrosion resistance and catalytic properties despite lower conductivity.
Environmental Impact and Sustainability
Palladium and copper alloys differ significantly in environmental impact and sustainability, with copper being more abundant and easier to recycle, resulting in a smaller ecological footprint. Palladium mining involves higher energy consumption and generates more toxic byproducts, making it less eco-friendly and posing challenges for sustainable sourcing. Choosing copper alloys supports circular economy principles due to their recyclability, while palladium's scarcity and extraction impact raise concerns for long-term environmental sustainability.
Conclusion: Choosing the Right Metal for Alloys
Palladium offers superior corrosion resistance, excellent catalytic properties, and a higher market value, making it ideal for high-performance and luxury alloys. Copper provides excellent electrical conductivity, affordability, and ease of alloying, commonly used in industrial and electrical applications. Selecting the right metal depends on balancing cost, desired physical properties, and application requirements, with palladium favored for precision and durability, while copper suits cost-effective, versatile uses.
Infographic: Palladium vs Copper for Alloy
 azmater.com
azmater.com