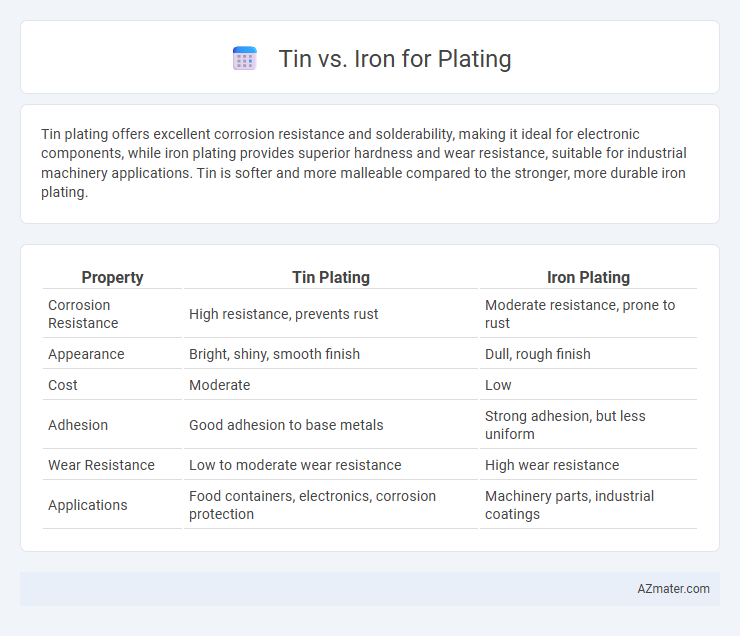
Tin plating offers excellent corrosion resistance and solderability, making it ideal for electronic components, while iron plating provides superior hardness and wear resistance, suitable for industrial machinery applications. Tin is softer and more malleable compared to the stronger, more durable iron plating.
Table of Comparison
| Property | Tin Plating | Iron Plating |
|---|---|---|
| Corrosion Resistance | High resistance, prevents rust | Moderate resistance, prone to rust |
| Appearance | Bright, shiny, smooth finish | Dull, rough finish |
| Cost | Moderate | Low |
| Adhesion | Good adhesion to base metals | Strong adhesion, but less uniform |
| Wear Resistance | Low to moderate wear resistance | High wear resistance |
| Applications | Food containers, electronics, corrosion protection | Machinery parts, industrial coatings |
Introduction to Metal Plating: Tin vs Iron
Tin and iron serve distinct functions in metal plating due to their unique properties. Tin plating offers excellent corrosion resistance, solderability, and a bright, smooth finish, making it ideal for electrical components and food packaging. Iron plating, though less common, provides increased hardness and wear resistance, often used in industrial machinery applications where durability is essential.
Chemical Properties of Tin and Iron
Tin exhibits excellent corrosion resistance due to the formation of a protective oxide layer, making it highly effective for plating applications that require durability against oxidation. Iron, on the other hand, is more prone to rusting as it forms iron oxide (rust) when exposed to moisture and oxygen, which compromises its protective qualities. The chemical stability of tin's oxide compared to the reactive nature of iron oxidation plays a crucial role in selecting tin over iron for plating to enhance longevity and resistance to environmental degradation.
Plating Process: Tin vs Iron
Tin plating offers superior corrosion resistance and excellent solderability, making it ideal for electronic components and food packaging applications. Iron plating, on the other hand, provides enhanced hardness and wear resistance, often used in automotive and industrial machinery parts. The plating process for tin involves electroplating from stannous salts, whereas iron plating typically uses an electroforming technique requiring precise control to prevent oxidation and ensure uniform coating.
Corrosion Resistance Comparison
Tin plating provides superior corrosion resistance in mildly acidic and neutral environments due to its ability to form a stable, protective oxide layer that prevents substrate oxidation. Iron plating, while harder and more wear-resistant, is prone to rust and corrosion when exposed to moisture and oxygen without proper protective coatings. Tin's non-toxic nature and excellent solderability make it the preferred choice for electronics and food-grade applications requiring long-term corrosion protection.
Electrical Conductivity Differences
Tin plating offers lower electrical conductivity compared to iron, with tin's conductivity at about 9.17 x 10^6 S/m versus iron's approximately 1.0 x 10^7 S/m. This difference impacts the efficiency of electrical connections, where iron plating provides better current flow and reduced energy loss. Applications requiring high conductivity often favor iron plating despite its susceptibility to corrosion relative to tin's superior corrosion resistance.
Cost and Availability of Tin and Iron
Tin plating offers cost advantages due to tin's relatively low price and abundant global supply from major producers like China and Indonesia, ensuring consistent availability. Iron plating, although cheaper per raw material unit, often incurs higher processing costs because of its susceptibility to rust and need for protective coatings. The widespread availability of tin and its corrosion-resistant properties make it a preferred choice for plating applications where cost-efficiency and durability are critical.
Applications in Industry
Tin plating provides excellent corrosion resistance and solderability, making it ideal for electronics, food packaging, and automotive components. Iron plating, often applied through electroplating or galvanizing, enhances surface hardness and wear resistance, commonly used in heavy machinery, tools, and construction equipment. Industrial applications prioritize tin for its non-toxic properties and iron for structural durability and cost-effectiveness.
Environmental Impact and Safety
Tin plating offers a lower environmental impact compared to iron plating due to its resistance to corrosion and non-toxic nature, reducing the need for frequent re-coating and chemical treatments. Iron plating often involves hazardous substances such as cyanide in the plating process, increasing environmental hazards and safety risks for workers. Choosing tin plating supports safer handling practices and promotes sustainability by minimizing toxic waste and ensuring safer disposal procedures.
Surface Finish and Aesthetics
Tin plating provides a bright, smooth surface finish with excellent corrosion resistance and a naturally shiny, silver-white appearance, making it ideal for decorative applications. Iron plating offers a rougher texture with a darker, more matte finish prone to rust without protective coatings, which limits its aesthetic appeal. Tin's superior adhesion and uniform coating ensure consistent, visually appealing surfaces preferred for consumer electronics, kitchenware, and automotive parts.
Choosing the Right Metal for Plating
Choosing the right metal for plating depends on factors such as corrosion resistance, conductivity, and cost, where tin and iron serve distinct purposes. Tin offers excellent corrosion resistance and solderability, making it ideal for electronics and food packaging applications, while iron provides superior strength and durability, suitable for automotive and heavy machinery parts. Evaluating environmental exposure and mechanical requirements ensures optimal plating performance and longevity.
Infographic: Tin vs Iron for Plating
 azmater.com
azmater.com