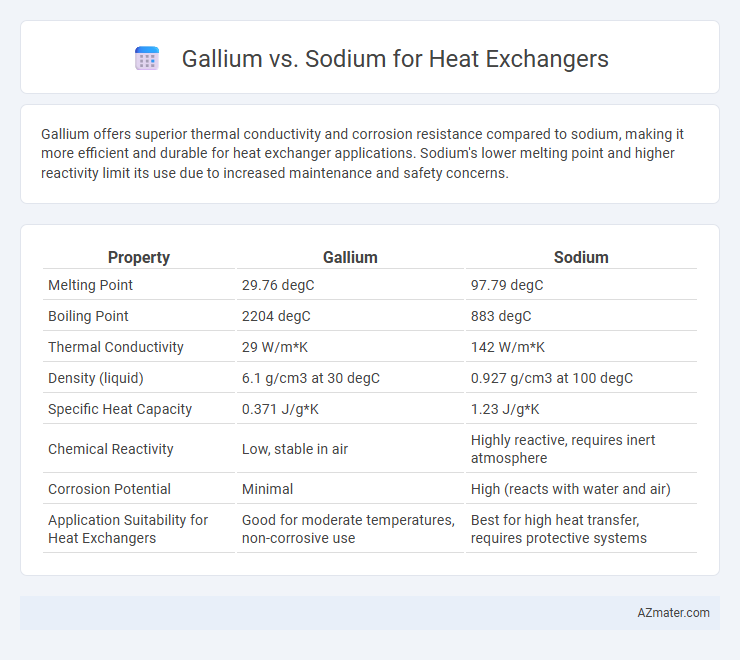
Gallium offers superior thermal conductivity and corrosion resistance compared to sodium, making it more efficient and durable for heat exchanger applications. Sodium's lower melting point and higher reactivity limit its use due to increased maintenance and safety concerns.
Table of Comparison
| Property | Gallium | Sodium |
|---|---|---|
| Melting Point | 29.76 degC | 97.79 degC |
| Boiling Point | 2204 degC | 883 degC |
| Thermal Conductivity | 29 W/m*K | 142 W/m*K |
| Density (liquid) | 6.1 g/cm3 at 30 degC | 0.927 g/cm3 at 100 degC |
| Specific Heat Capacity | 0.371 J/g*K | 1.23 J/g*K |
| Chemical Reactivity | Low, stable in air | Highly reactive, requires inert atmosphere |
| Corrosion Potential | Minimal | High (reacts with water and air) |
| Application Suitability for Heat Exchangers | Good for moderate temperatures, non-corrosive use | Best for high heat transfer, requires protective systems |
Introduction to Heat Exchanger Materials
Gallium and sodium are two advanced heat exchanger materials offering distinct thermal properties crucial for high-efficiency heat transfer systems. Gallium boasts a high thermal conductivity of approximately 29 W/m*K and remains liquid near room temperature, providing corrosion resistance and stability in moderate temperature applications. Sodium, with an exceptional thermal conductivity around 142 W/m*K and a melting point of 98degC, excels in high-temperature environments such as nuclear reactors, though it requires careful handling due to its chemical reactivity.
Overview of Gallium as a Heat Transfer Medium
Gallium offers exceptional thermal conductivity and a wide liquid temperature range from 29.76degC (melting point) to over 2200degC (boiling point), making it highly effective for heat exchangers in high-temperature applications. Unlike sodium, gallium is non-reactive with air and water, reducing corrosion risks and enhancing system longevity. Its low vapor pressure and non-toxicity provide safer handling and operational benefits in advanced thermal management systems.
Overview of Sodium as a Heat Transfer Medium
Sodium, a highly efficient heat transfer medium, offers exceptional thermal conductivity and a high boiling point, making it ideal for high-temperature heat exchangers in industrial and nuclear applications. Its low density and excellent heat capacity allow for rapid and uniform heat distribution, reducing thermal stress on equipment. However, sodium's vigorous chemical reactivity with water and air requires specialized handling and containment systems to ensure operational safety.
Thermal Conductivity: Gallium vs Sodium
Gallium exhibits a thermal conductivity of approximately 29 W/m*K, which is significantly lower than sodium's thermal conductivity of around 142 W/m*K, making sodium a superior conductor of heat in heat exchanger applications. Sodium's high thermal conductivity facilitates efficient heat transfer and rapid temperature equalization, critical in high-performance cooling systems like nuclear reactors. Despite gallium's lower thermal conductivity, its non-reactive nature and liquid state near room temperature offer advantages in specific low-temperature or chemically sensitive environments.
Operating Temperature Ranges
Gallium offers a wider operating temperature range for heat exchangers, remaining stable from approximately 29.8degC to over 2200degC, making it suitable for high-temperature applications. Sodium, often used in liquid metal heat exchangers, operates effectively between 98degC and 883degC, with excellent thermal conductivity and low viscosity but has limitations due to its lower melting point and reactivity. Selecting gallium or sodium depends on the specific operating temperature requirements and chemical compatibility of the heat exchanger system.
Chemical Stability and Reactivity
Gallium exhibits superior chemical stability compared to sodium, maintaining a non-reactive state with common materials used in heat exchangers and resisting oxidation under normal operating conditions. Sodium, while highly efficient as a heat transfer fluid due to its excellent thermal conductivity, is extremely reactive with water and air, posing significant challenges in terms of corrosion and safety. The chemical inertness of gallium reduces the risk of material degradation and system failure, making it a more reliable choice for long-term heat exchanger applications where stability is critical.
Safety Considerations and Risks
Gallium offers lower toxicity and reduced flammability compared to sodium, making it a safer choice for heat exchanger applications where human exposure and fire hazards are concerns. Sodium's high reactivity with water and air presents significant explosion and combustion risks, requiring rigorous handling protocols and inert atmospheres. The stability of gallium in ambient conditions minimizes corrosion and containment challenges, enhancing operational safety and reducing potential environmental impacts.
Compatibility with Common Heat Exchanger Designs
Gallium exhibits superior compatibility with common heat exchanger designs due to its low vapor pressure and non-corrosive behavior with metals such as stainless steel and copper, making it ideal for closed-loop systems. Sodium, while offering excellent thermal conductivity, poses significant challenges including high reactivity with water and oxygen, necessitating specialized materials like nickel-based alloys and protective atmospheres to prevent corrosion and safety hazards. Gallium's stable liquid phase near room temperature also allows for simpler heat exchanger fabrication and maintenance compared to sodium's high melting point and aggressive chemical nature.
Cost and Availability
Gallium is significantly more expensive than sodium, with prices often exceeding hundreds of dollars per kilogram due to its rarity and extraction complexity. Sodium, abundant in nature and produced on a large scale, remains far more cost-effective for heat exchanger applications. The high availability of sodium ensures consistent supply and lower operational costs compared to the limited and costly gallium resources.
Final Comparison: Which is Superior for Heat Exchangers?
Gallium offers superior heat transfer efficiency, higher thermal conductivity (approximately 29 W/m*K), and excellent corrosion resistance compared to sodium, which has a thermal conductivity of about 142 W/m*K but poses safety challenges due to its high reactivity with water and air. Sodium's low melting point (-98degC) allows efficient operation at lower temperatures, making it ideal for high-temperature reactors despite requiring stringent handling protocols. For most industrial heat exchanger applications prioritizing safety, stability, and moderate thermal performance, gallium is preferred, while sodium excels in specialized high-temperature environments demanding maximum thermal conductivity.
Infographic: Gallium vs Sodium for Heat Exchanger
 azmater.com
azmater.com