Osmium, a dense and stable transition metal, is widely used in high-precision research due to its durability and resistance to corrosion. Hassium, a synthetic and highly radioactive element with a short half-life, poses challenges for research but offers potential insights into superheavy element chemistry.
Table of Comparison
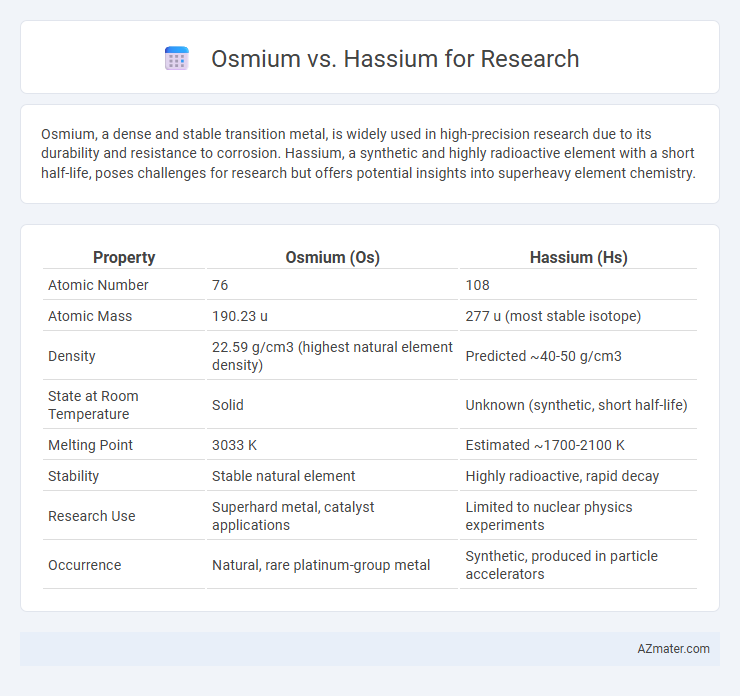
| Property | Osmium (Os) | Hassium (Hs) |
|---|---|---|
| Atomic Number | 76 | 108 |
| Atomic Mass | 190.23 u | 277 u (most stable isotope) |
| Density | 22.59 g/cm3 (highest natural element density) | Predicted ~40-50 g/cm3 |
| State at Room Temperature | Solid | Unknown (synthetic, short half-life) |
| Melting Point | 3033 K | Estimated ~1700-2100 K |
| Stability | Stable natural element | Highly radioactive, rapid decay |
| Research Use | Superhard metal, catalyst applications | Limited to nuclear physics experiments |
| Occurrence | Natural, rare platinum-group metal | Synthetic, produced in particle accelerators |
Introduction to Osmium and Hassium
Osmium, a dense transition metal with atomic number 76, is valued for its high melting point, hardness, and durability in research applications. Hassium, element 108, is a synthetic superheavy element produced in particle accelerators, primarily studied to explore nuclear stability and relativistic effects. Comparative research highlights osmium's practical material uses versus hassium's role in advancing theoretical nuclear chemistry.
Historical Discovery of Osmium and Hassium
Osmium was discovered in 1803 by Smithson Tennant during the analysis of platinum ore, characterized by its high density and distinctive blue-gray color, making it significant in early transition metal research. Hassium, on the other hand, was first synthesized in 1984 at the GSI Helmholtz Centre for Heavy Ion Research in Germany by bombarding lead-208 with iron-58 ions, representing one of the heaviest elements created artificially. The contrasting discovery methods highlight osmium's natural occurrence and hassium's role in advancing nuclear chemistry and superheavy element studies.
Chemical Properties Comparison
Osmium and Hassium, both transition metals, exhibit distinct chemical properties critical for research applications; Osmium, a dense, naturally occurring element with a high oxidation state of +8, demonstrates remarkable catalytic activity and corrosion resistance in chemical reactions. Hassium, a synthetic, highly radioactive element with a similar group placement but limited practical data due to its short half-life, shows predicted chemical behavior resembling Osmium's, particularly in forming volatile hexafluorides, useful for understanding superheavy element chemistry. Researching their comparative chemical properties enhances knowledge of periodic trends and stability within group 8 elements, advancing inorganic chemistry and nuclear science.
Physical Characteristics Overview
Osmium, a dense, blue-gray transition metal with a melting point of 3045degC and a density of 22.59 g/cm3, is extensively used for high-precision instruments due to its hardness and durability. Hassium, a synthetic element with atomic number 108, has a very short half-life and limited available data on its physical properties, but it is predicted to be a dense, heavy metal similar to osmium in group 8 of the periodic table. Research on osmium focuses on its stable isotopes and well-documented physical characteristics, while hassium remains primarily of theoretical and experimental interest due to its rapid radioactive decay and scarcity.
Abundance and Availability
Osmium, one of the densest naturally occurring elements, has an average crustal abundance of about 0.001 ppm, making it scarce but accessible for research through mining of platinum ores. Hassium, a synthetic element with atomic number 108, does not occur naturally and can only be produced in minute quantities in particle accelerators, severely limiting its availability for extensive experimental studies. The significantly greater abundance and accessibility of osmium facilitate a broader scope of research compared to the highly restricted production and availability of hassium.
Applications in Scientific Research
Osmium, a dense transition metal, is widely utilized in applications such as electron microscopy sample preparation and high-precision electrical contacts due to its hardness and stability. Hassium, a synthetic element with a very short half-life, has limited practical uses but remains valuable in nuclear chemistry experiments to study transactinide properties and superheavy element behavior. Research on both elements advances understanding in materials science and nuclear physics, with osmium offering established applications and hassium contributing to theoretical explorations.
Safety and Handling Considerations
Osmium is a dense, naturally occurring element with established handling protocols due to its toxicity primarily from osmium tetroxide exposure, requiring meticulous ventilation and protective equipment in research settings. Hassium, a synthetic, highly radioactive element produced in minute quantities, poses significant radiological hazards and extreme instability, limiting practical handling to specialized facilities equipped with remote manipulation tools and stringent containment procedures. Safety in research involving osmium centers on chemical toxicity control, whereas hassium demands rigorous radiation shielding and strict adherence to nuclear safety regulations.
Cost and Sourcing Challenges
Osmium, a dense platinum-group metal, is relatively more accessible with established mining and refining processes, whereas hassium is a synthetic element produced only in particle accelerators, making it extremely scarce and costly to obtain. Osmium's cost is high due to its rarity and complex extraction, but it remains feasible for research applications. Hassium's practical use in research is limited by its half-life of mere seconds and the exorbitant expense and difficulty associated with its production in minute quantities.
Future Prospects in Research
Osmium, a dense transition metal with stable isotopes, remains crucial in catalysis, material science, and high-pressure physics due to its unique electronic properties and durability. Hassium, a synthetic superheavy element with atomic number 108, offers potential insights into the "island of stability," nuclear structure, and relativistic effects, though its short half-life limits practical experimentation. Future research aims to synthesize longer-lived hassium isotopes, enabling detailed chemical studies and expanding understanding of superheavy element behavior compared to well-characterized osmium.
Conclusion: Choosing the Right Element
Osmium offers practical advantages in research due to its high density, stability, and availability, making it suitable for applications in catalysis, electronics, and material science. Hassium, a synthetic element with a short half-life and limited availability, presents significant challenges for experimental studies, restricting its use primarily to nuclear physics research. Selecting the right element depends on research goals; osmium is preferred for applied sciences, while hassium is targeted for theoretical and nuclear structure investigations.
Infographic: Osmium vs Hassium for Research
 azmater.com
azmater.com