Gallium alloys offer low melting points and excellent wettability, making them ideal for flexible electronics and thermal interface materials. Chromium alloys provide superior corrosion resistance and high-temperature strength, commonly used in aerospace and industrial applications.
Table of Comparison
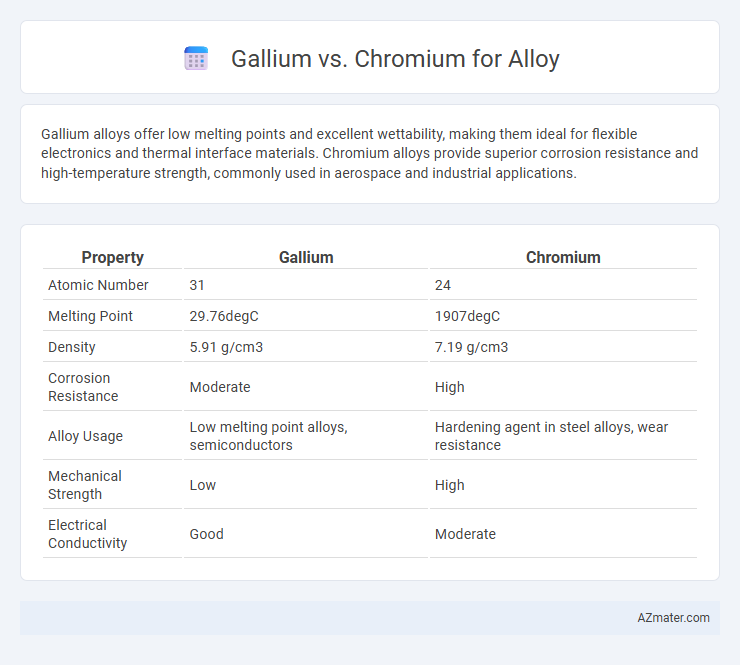
| Property | Gallium | Chromium |
|---|---|---|
| Atomic Number | 31 | 24 |
| Melting Point | 29.76degC | 1907degC |
| Density | 5.91 g/cm3 | 7.19 g/cm3 |
| Corrosion Resistance | Moderate | High |
| Alloy Usage | Low melting point alloys, semiconductors | Hardening agent in steel alloys, wear resistance |
| Mechanical Strength | Low | High |
| Electrical Conductivity | Good | Moderate |
Introduction to Gallium and Chromium in Alloys
Gallium and chromium are critical elements in alloy production, each offering unique properties that enhance metal performance. Gallium improves alloy malleability and low melting points, making it valuable for specialized applications such as semiconductors and thermometers. Chromium contributes excellent corrosion resistance and hardness, commonly used in stainless steel alloys to increase durability and wear resistance.
Chemical Properties Relevant to Alloy Formation
Gallium exhibits a low melting point of 29.76degC and forms intermetallic compounds with metals, enhancing alloy malleability and conductivity. Chromium, with a melting point of 1907degC, contributes high corrosion resistance and hardness due to its stable oxide layer, making it ideal for stainless steel alloys. The contrasting chemical properties of gallium's low melting and reactivity and chromium's oxidation resistance are critical in tailoring alloy characteristics for diverse industrial applications.
Mechanical Strength: Gallium vs Chromium Alloys
Chromium alloys exhibit superior mechanical strength compared to gallium alloys, making them ideal for high-stress applications such as aerospace and automotive components. Gallium alloys, while offering unique low-melting properties and excellent corrosion resistance, typically lack the tensile strength and hardness found in chromium-based materials. The intrinsic atomic structure of chromium contributes to higher yield strength and durability, whereas gallium's softness limits its use in load-bearing alloy compositions.
Melting Point and Thermal Stability Comparison
Gallium alloys exhibit a melting point around 29.8degC, significantly lower than chromium's melting point of 1907degC, making gallium beneficial for low-temperature applications and fusible alloy formulations. Chromium demonstrates superior thermal stability and oxidation resistance at high temperatures, ideal for high-performance alloys in extreme environments. The choice between gallium and chromium alloys depends on balancing the need for low melting points versus high thermal endurance in aerospace, electronics, and corrosion-resistant materials.
Corrosion Resistance in Gallium and Chromium Alloys
Gallium alloys exhibit unique corrosion resistance due to the element's tendency to form a stable oxide layer that protects the base metal, particularly in low-temperature applications and in environments with mild oxidizers. Chromium alloys are renowned for their superior corrosion resistance, primarily because chromium forms a highly adherent and durable chromium oxide film that prevents further surface degradation, making these alloys ideal for harsh environments and high-temperature applications. Comparative studies show that chromium alloys generally outperform gallium alloys in corrosive durability, especially in aggressive chemical or high-temperature conditions, although gallium alloys offer specialized advantages in coating and soldering applications.
Industrial Applications: Where Each Excels
Gallium alloys excel in semiconductor and LED manufacturing due to their excellent electrical conductivity and low melting points, offering precision in electronic components. Chromium alloys dominate in corrosion-resistant applications, such as stainless steel production, because of their hardness, high melting point, and oxidation resistance. Industrial sectors requiring wear resistance and structural integrity often prefer chromium-based alloys, while specialized electronics benefit from gallium's unique properties.
Environmental Impact and Sustainability
Gallium alloys demonstrate a lower environmental impact compared to chromium alloys due to gallium's relative abundance and non-toxic nature, reducing hazardous waste concerns during production and recycling. Chromium mining and alloy processing involve significant ecological risks, including soil and water contamination from hexavalent chromium compounds, which are highly toxic and carcinogenic. Sustainable applications favor gallium alloys for their recyclability and lower lifecycle emissions, making them more environmentally responsible choices in advanced materials development.
Cost and Availability Factors
Gallium, known for its rarity and limited production primarily from bauxite processing, tends to be more expensive than chromium, which is abundant and extracted extensively from chromite ore deposits worldwide. Chromium's widespread availability and lower cost make it a preferred choice for large-scale alloy manufacturing, especially in stainless steel and superalloys. Gallium alloys, despite higher costs, offer unique properties such as low melting points and superior conductivity, valuable in specialized applications but less common in mass alloy production.
Alloying Behavior with Common Metals
Gallium exhibits low melting points and a strong tendency to form intermetallic compounds with metals like aluminum, making it effective in enhancing ductility and corrosion resistance in alloys. Chromium's alloying behavior is characterized by its ability to improve hardness, wear resistance, and oxidation resistance, especially when combined with iron, nickel, and stainless steel matrices. Compared to chromium, gallium generally forms more brittle phases with common metals, while chromium stabilizes solid solutions, contributing to superior mechanical strength in alloy systems.
Future Trends in Gallium and Chromium Alloy Research
Future trends in gallium and chromium alloy research emphasize gallium's role in enhancing thermal and electrical conductivity in high-performance electronics, while chromium continues to dominate corrosion resistance and hardness in structural alloys. Emerging gallium-based alloys show promise in flexible electronics and low-temperature soldering applications, driving innovation in wearable technology and microelectronics. Research into chromium alloys focuses on nano-scale structuring to improve strength-to-weight ratios for aerospace and automotive sectors.
Infographic: Gallium vs Chromium for Alloy
 azmater.com
azmater.com