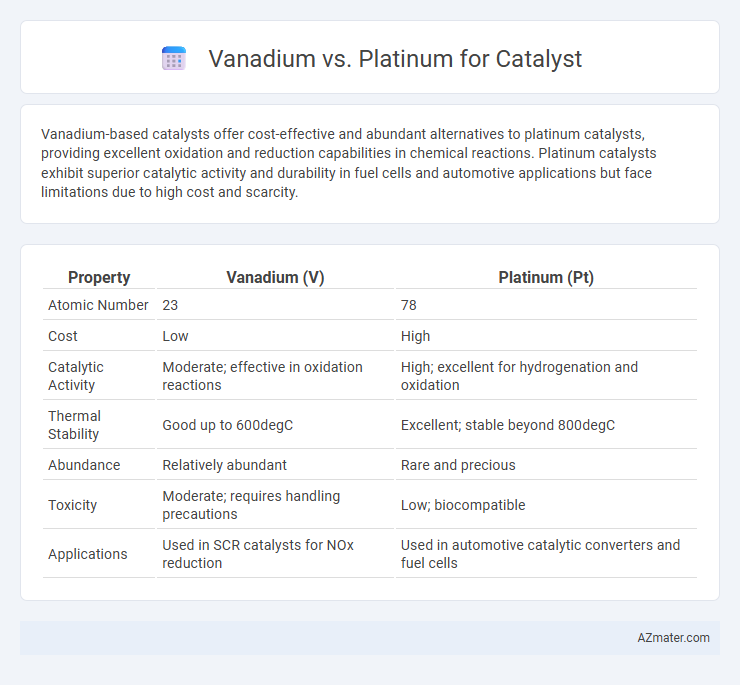
Vanadium-based catalysts offer cost-effective and abundant alternatives to platinum catalysts, providing excellent oxidation and reduction capabilities in chemical reactions. Platinum catalysts exhibit superior catalytic activity and durability in fuel cells and automotive applications but face limitations due to high cost and scarcity.
Table of Comparison
| Property | Vanadium (V) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 23 | 78 |
| Cost | Low | High |
| Catalytic Activity | Moderate; effective in oxidation reactions | High; excellent for hydrogenation and oxidation |
| Thermal Stability | Good up to 600degC | Excellent; stable beyond 800degC |
| Abundance | Relatively abundant | Rare and precious |
| Toxicity | Moderate; requires handling precautions | Low; biocompatible |
| Applications | Used in SCR catalysts for NOx reduction | Used in automotive catalytic converters and fuel cells |
Introduction to Vanadium and Platinum Catalysts
Vanadium catalysts, known for their high oxidation states and variable valence, play a crucial role in oxidation reactions such as sulfuric acid production and selective oxidation of hydrocarbons. Platinum catalysts exhibit exceptional activity and stability in hydrogenation and reforming processes, widely utilized in automotive catalytic converters and chemical synthesis. Both metals offer distinct advantages: vanadium provides cost-effective catalytic oxidation, while platinum delivers superior performance in hydrogenation and dehydrogenation reactions.
Chemical Properties: Vanadium vs Platinum
Vanadium exhibits multiple oxidation states ranging from +2 to +5, enabling versatile catalytic functions in oxidation reactions, while platinum primarily maintains a stable +2 or +4 state, favoring hydrogenation and dehydrogenation processes. Vanadium oxides provide strong acidity and redox properties, making them effective in selective oxidation and sulfuric acid production, whereas platinum offers superior resistance to sintering and high thermal stability critical in automotive catalytic converters. The differing electronic configurations and surface chemistries of vanadium and platinum significantly influence their catalytic activity, selectivity, and durability under varied chemical environments.
Catalytic Efficiency Comparison
Vanadium-based catalysts exhibit strong oxidative capabilities and high selectivity in partial oxidation reactions, making them efficient for applications such as sulfur removal and oxidation processes. Platinum catalysts demonstrate superior catalytic efficiency in hydrogenation and dehydrogenation reactions due to their exceptional activity and stability at lower temperatures. Comparative studies show platinum typically outperforms vanadium in terms of turnover frequency and durability in hydrogenation, while vanadium excels in oxidation reactions, highlighting a catalytic efficiency difference driven by reaction type and process conditions.
Industrial Applications of Vanadium and Platinum
Vanadium catalysts excel in sulfuric acid production and selective oxidation processes, offering cost-effective and durable solutions for large-scale industrial applications. Platinum catalysts dominate in automotive catalytic converters and fuel cells due to their superior activity and resistance to poisoning, despite higher costs and limited availability. The choice between vanadium and platinum hinges on balancing catalytic efficiency, operating conditions, and economic factors in industries such as chemical manufacturing, energy, and environmental protection.
Cost Analysis: Vanadium vs Platinum Catalysts
Vanadium catalysts offer a significantly lower cost compared to platinum catalysts, making them an economically attractive option for large-scale industrial processes such as sulfuric acid production and selective oxidation reactions. The price of platinum fluctuates around $30,000 per kilogram due to its rarity and extensive industrial demand, whereas vanadium costs approximately $50 per kilogram, providing substantial savings in catalyst expenditures. Despite platinum's higher catalytic efficiency and durability, the cost-effectiveness of vanadium catalysts makes them favorable for applications where budget constraints and scalability are critical.
Environmental Impact and Sustainability
Vanadium catalysts demonstrate lower environmental impact compared to platinum by utilizing more abundant resources, which reduces mining and extraction-related ecological damage. Vanadium's higher corrosion resistance and recyclability contribute to sustainability through extended catalyst lifespan and decreased waste generation. Platinum, though highly effective, involves energy-intensive production and limited global reserves, posing challenges for long-term sustainable catalyst development.
Durability and Longevity in Catalytic Processes
Vanadium catalysts demonstrate high thermal stability and resistance to sintering, enhancing durability in oxidation reactions, but may undergo gradual deactivation due to phase transformations. Platinum catalysts exhibit superior chemical stability and resistance to poisoning, resulting in prolonged longevity in hydrogenation and reforming processes. Despite platinum's higher cost, its exceptional durability under diverse catalytic conditions often makes it the preferred choice for long-term industrial applications.
Reaction Specificity and Selectivity
Vanadium catalysts exhibit high reaction specificity, particularly in partial oxidation and selective catalytic reduction processes, due to their variable oxidation states that facilitate precise electron transfer. Platinum catalysts offer superior selectivity in hydrogenation and dehydrogenation reactions, attributed to their ability to adsorb reactants with controlled activation energy, minimizing side reactions. Comparative studies highlight vanadium's advantage in oxidation environments, while platinum excels in hydrogen-friendly conditions, dictating catalyst choice based on targeted reaction pathways.
Emerging Trends in Catalyst Technology
Vanadium catalysts are gaining traction in emerging catalyst technology due to their cost-effectiveness and high oxidative stability for selective oxidation reactions, particularly in the production of sulfuric acid and organic chemicals. Platinum catalysts remain essential for hydrogenation and oxidation processes, offering superior activity and durability but at a significantly higher cost and susceptibility to poisoning from sulfur compounds. Recent research focuses on hybrid catalysts combining vanadium's affordability and platinum's catalytic efficiency to enhance performance and sustainability in industrial applications.
Choosing the Right Catalyst: Key Considerations
Vanadium catalysts offer cost-effective oxidation benefits and are highly effective in selective oxidation reactions, making them ideal for large-scale industrial processes, while platinum catalysts provide superior durability and exceptional activity in hydrogenation and reforming reactions. Selecting the right catalyst depends on factors such as reaction type, temperature stability, resistance to poisoning, and economic considerations including raw material cost and catalyst lifespan. Evaluating the specific chemical environment and performance requirements ensures optimal catalyst choice between vanadium's oxidation strength and platinum's catalytic precision.
Infographic: Vanadium vs Platinum for Catalyst
 azmater.com
azmater.com