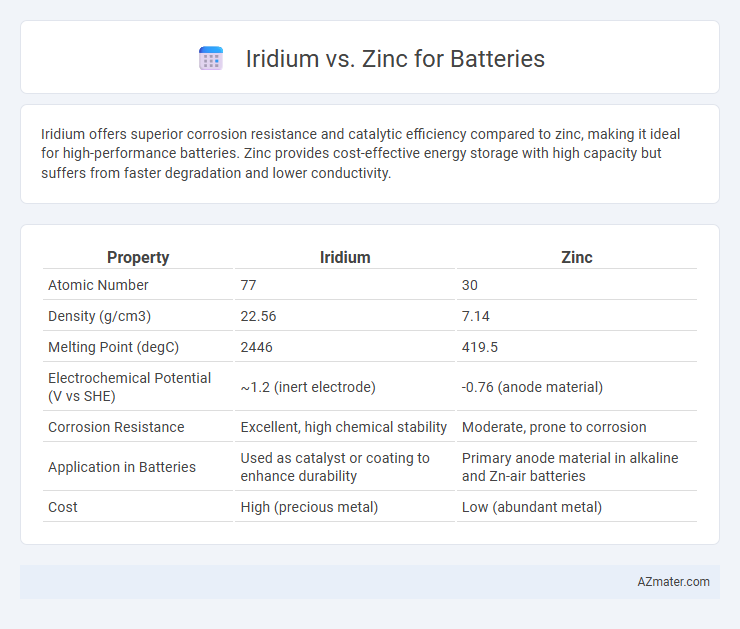
Iridium offers superior corrosion resistance and catalytic efficiency compared to zinc, making it ideal for high-performance batteries. Zinc provides cost-effective energy storage with high capacity but suffers from faster degradation and lower conductivity.
Table of Comparison
| Property | Iridium | Zinc |
|---|---|---|
| Atomic Number | 77 | 30 |
| Density (g/cm3) | 22.56 | 7.14 |
| Melting Point (degC) | 2446 | 419.5 |
| Electrochemical Potential (V vs SHE) | ~1.2 (inert electrode) | -0.76 (anode material) |
| Corrosion Resistance | Excellent, high chemical stability | Moderate, prone to corrosion |
| Application in Batteries | Used as catalyst or coating to enhance durability | Primary anode material in alkaline and Zn-air batteries |
| Cost | High (precious metal) | Low (abundant metal) |
Introduction to Iridium and Zinc in Battery Technology
Iridium and zinc represent two distinct approaches in battery technology, with iridium mainly used in high-performance electrodes due to its exceptional corrosion resistance and catalytic properties, while zinc is favored for its abundance, low cost, and high energy density in aqueous battery systems like zinc-air batteries. Iridium's role is crucial in enhancing the durability and efficiency of fuel cells and certain electrochemical cells, whereas zinc's versatility supports large-scale energy storage and environmentally friendly battery solutions. Advances in material science have focused on optimizing iridium-based catalysts and improving zinc electrode stability to boost battery performance and lifespan.
Chemical Properties: Iridium vs Zinc
Iridium exhibits exceptional chemical stability and corrosion resistance, making it highly durable in harsh electrochemical environments, while zinc is more reactive, readily oxidizing and forming zinc oxide during battery operation. The high melting point and low electrochemical potential of iridium contribute to its robustness in catalyzing oxidation-reduction reactions, contrasting with zinc's lower melting point and higher reactivity that favor its use in primary batteries. These fundamental chemical properties impact the electrode lifespan and performance, with iridium offering long-term stability and zinc providing cost-effective but less durable energy storage solutions.
Electrochemical Performance Differences
Iridium batteries exhibit superior electrochemical performance with higher catalytic activity and better stability in oxygen evolution and reduction reactions compared to zinc batteries, which primarily rely on zinc metal's high energy density and cost-effectiveness but suffer from dendrite formation and limited cycle life. Iridium's corrosion resistance and ability to facilitate fast electron transfer contribute to enhanced cycle stability and efficiency, essential for advanced applications like fuel cells and water electrolysis. Zinc batteries, while offering high theoretical capacity and environmental benefits, lag in electrochemical kinetics and often require additives or complex designs to improve rechargeability and maintain performance over multiple cycles.
Energy Density Comparison
Iridium-based batteries typically offer higher energy density compared to zinc batteries due to iridium's superior electrochemical properties and ability to facilitate more efficient electron transfer. Zinc batteries, while generally safer and more cost-effective, exhibit lower energy density, limiting their use in high-capacity energy storage applications. Energy density in iridium electrodes can reach values exceeding 300 Wh/kg, significantly outperforming zinc's typical range of 100-150 Wh/kg.
Cost Analysis: Iridium vs Zinc
Iridium batteries have significantly higher costs due to the rarity and expense of iridium metal, with prices often exceeding $6,000 per ounce, making large-scale production economically challenging. Zinc batteries benefit from zinc's abundance and low cost, typically priced under $2 per pound, enabling more affordable battery manufacturing. Cost analysis clearly favors zinc batteries for applications requiring cost-effectiveness and scalability, while iridium's high cost limits its use to niche, high-performance energy storage solutions.
Environmental Impact and Sustainability
Iridium, a rare and expensive metal, shows limited environmental sustainability due to its scarce natural reserves and energy-intensive extraction processes. Zinc, abundant and more environmentally benign, offers enhanced sustainability for battery production through its widespread availability and recyclability, leading to lower ecological footprints and reduced toxic waste generation. Zinc-based batteries contribute significantly to greener energy storage solutions, making them preferable for sustainable technology development compared to iridium-dependent systems.
Applications in Modern Battery Systems
Iridium enhances battery performance through its high corrosion resistance and catalytic properties, making it ideal for advanced energy storage such as fuel cells and hybrid batteries. Zinc, valued for its abundance, low cost, and environmentally friendly profile, is commonly used in zinc-air and zinc-carbon batteries for portable electronics and grid storage. Modern battery systems leverage iridium for longevity and efficiency in high-demand applications, while zinc provides a sustainable alternative for scalable and cost-effective energy solutions.
Lifecycle and Durability
Iridium-based batteries exhibit superior lifecycle performance compared to zinc batteries, often enduring thousands of charge-discharge cycles with minimal capacity degradation. Zinc batteries, while cost-effective and environmentally friendly, typically have shorter lifespans due to dendrite formation and lower chemical stability. The durability of iridium compounds in battery electrodes significantly enhances resistance to corrosion and structural breakdown, extending overall battery longevity in demanding applications.
Safety Considerations
Iridium, while expensive, offers superior catalytic stability and corrosion resistance in battery electrodes, significantly reducing risks of thermal runaway and electrolyte decomposition compared to zinc. Zinc batteries are more prone to dendrite formation and hydrogen evolution, which can lead to short circuits and gas buildup, posing safety hazards during high discharge rates or prolonged use. Implementing iridium-based catalysts enhances battery safety by maintaining electrode integrity and minimizing hazardous side reactions.
Future Trends and Innovations
Iridium-based catalysts are emerging as a key innovation in battery technology due to their exceptional stability and efficiency in oxygen evolution reactions, crucial for next-generation metal-air and flow batteries. Zinc batteries continue to advance with enhanced anode designs and electrolyte formulations, offering low-cost, safe, and environmentally friendly energy storage solutions for large-scale applications. Future trends indicate a hybrid approach, integrating iridium catalysts in zinc-air battery systems to optimize performance, lifespan, and sustainability for grid and portable energy storage innovations.
Infographic: Iridium vs Zinc for Battery
 azmater.com
azmater.com