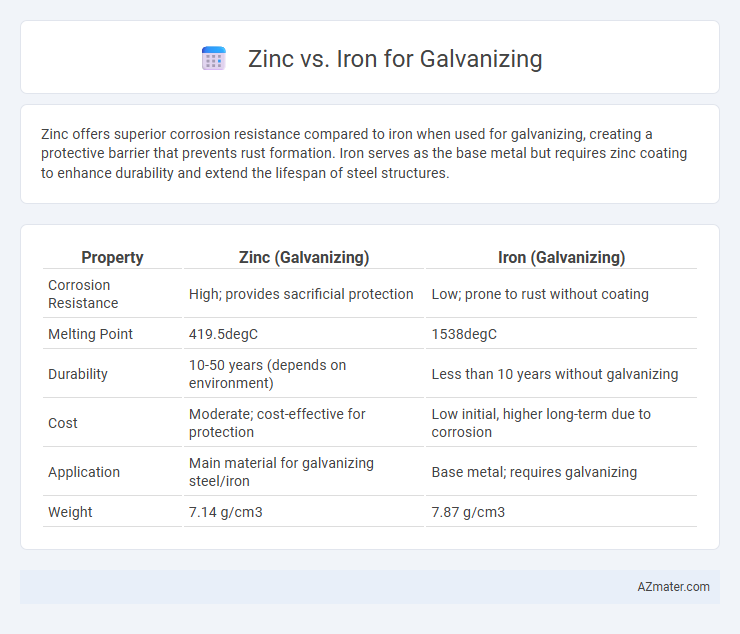
Zinc offers superior corrosion resistance compared to iron when used for galvanizing, creating a protective barrier that prevents rust formation. Iron serves as the base metal but requires zinc coating to enhance durability and extend the lifespan of steel structures.
Table of Comparison
| Property | Zinc (Galvanizing) | Iron (Galvanizing) |
|---|---|---|
| Corrosion Resistance | High; provides sacrificial protection | Low; prone to rust without coating |
| Melting Point | 419.5degC | 1538degC |
| Durability | 10-50 years (depends on environment) | Less than 10 years without galvanizing |
| Cost | Moderate; cost-effective for protection | Low initial, higher long-term due to corrosion |
| Application | Main material for galvanizing steel/iron | Base metal; requires galvanizing |
| Weight | 7.14 g/cm3 | 7.87 g/cm3 |
Introduction to Galvanizing: Purpose and Methods
Galvanizing involves coating steel or iron with a protective layer to prevent corrosion, with zinc being the most common metal used due to its superior sacrificial anode properties. Iron-based galvanizing methods, such as iron phosphating, provide surface preparation but lack the long-lasting protective qualities of zinc coatings. Hot-dip galvanizing uses molten zinc to create a durable, corrosion-resistant barrier that extends the lifespan of steel structures significantly.
Zinc vs Iron: Chemical Properties Overview
Zinc exhibits greater corrosion resistance than iron due to its ability to form a stable, adherent oxide layer that protects underlying metal from oxidation. Iron, when exposed to moisture and oxygen, readily forms rust (iron oxide), which is porous and flakes off, leading to continuous corrosion. The electrochemical potential difference between zinc and iron enables zinc to act as a sacrificial anode, providing galvanic protection by corroding preferentially and preserving the integrity of the iron substrate.
Corrosion Resistance: How Zinc and Iron Compare
Zinc offers superior corrosion resistance compared to iron due to its ability to form a stable, protective oxide layer that prevents rust formation. Iron, when exposed to moisture and oxygen, corrodes more rapidly, leading to rust and structural degradation. Galvanizing iron with zinc significantly extends the lifespan of the metal by providing a sacrificial barrier against corrosion.
Cost Analysis: Zinc vs Iron in Galvanizing
Zinc remains the preferred metal for galvanizing due to its superior corrosion resistance and cost-effectiveness compared to iron. Although iron is cheaper per unit weight, its lack of protective properties leads to higher long-term maintenance costs and shorter lifespan for galvanized products. The initial investment in zinc galvanizing ultimately offers a more economical solution by minimizing repair expenses and extending durability.
Environmental Impact: Zinc and Iron Effects
Zinc galvanized coatings offer superior corrosion resistance compared to iron, reducing the frequency of maintenance and replacement, which minimizes environmental waste. Zinc is more abundant and recyclable with a lower environmental footprint during extraction and processing compared to iron. However, zinc runoff can lead to localized soil and water contamination, while iron corrosion products generally pose less toxicity but degrade structural integrity faster, leading to increased resource consumption.
Durability and Longevity: Performance Comparison
Zinc offers superior corrosion resistance due to its sacrificial properties, providing durable protection for steel through galvanizing that can last up to 50 years in typical environmental conditions. Iron coatings, while less common for galvanizing, tend to offer limited corrosion resistance and shorter longevity, often under 20 years, due to their susceptibility to rust and oxidation. Zinc's self-healing ability and robust barrier protection make it the preferred choice for long-term durability and performance in galvanizing applications.
Application Areas: Where Zinc or Iron Works Best
Zinc excels in corrosion protection for outdoor structures such as bridges, automotive bodies, and electrical towers due to its sacrificial anode properties, making it ideal for harsh environmental conditions. Iron, primarily used in steel frameworks and heavy machinery, is favored in applications requiring superior tensile strength and load-bearing capacity but lacks the self-healing corrosion resistance of zinc. Galvanized zinc coatings outperform iron in marine, industrial, and infrastructure applications where durability against rust and chemical exposure is critical.
Maintenance Requirements for Zinc and Iron Galvanizing
Zinc galvanizing requires minimal maintenance due to its self-healing properties, where zinc corrosion products form a protective barrier that prevents rust. Iron galvanizing, typically achieved through steel coatings, demands more frequent inspections and touch-ups as it lacks zinc's sacrificial corrosion protection. Regular maintenance of iron surfaces often involves cleaning, painting, or reapplying protective coatings to prevent rust and extend longevity.
Safety Considerations: Handling Zinc and Iron
Handling zinc and iron during galvanizing requires strict adherence to safety protocols due to potential health hazards and fire risks. Zinc fumes released at high temperatures can cause metal fume fever, necessitating adequate ventilation and respiratory protection, while iron poses less inhalation risk but demands precautions against dust and sparks. Proper personal protective equipment (PPE), including gloves, goggles, and respirators, alongside safe storage and handling practices, are essential to minimize exposure and ensure workplace safety.
Conclusion: Choosing the Right Metal for Galvanizing
Zinc remains the preferred metal for galvanizing due to its excellent corrosion resistance, cost-effectiveness, and ease of application in protective coatings. While iron can be galvanized, it primarily serves as the base metal rather than the galvanizing agent, highlighting zinc's superior role in preventing rust. For optimal durability and long-term protection, selecting zinc as the galvanizing metal ensures enhanced performance and extended lifespan of steel structures.
Infographic: Zinc vs Iron for Galvanizing
 azmater.com
azmater.com