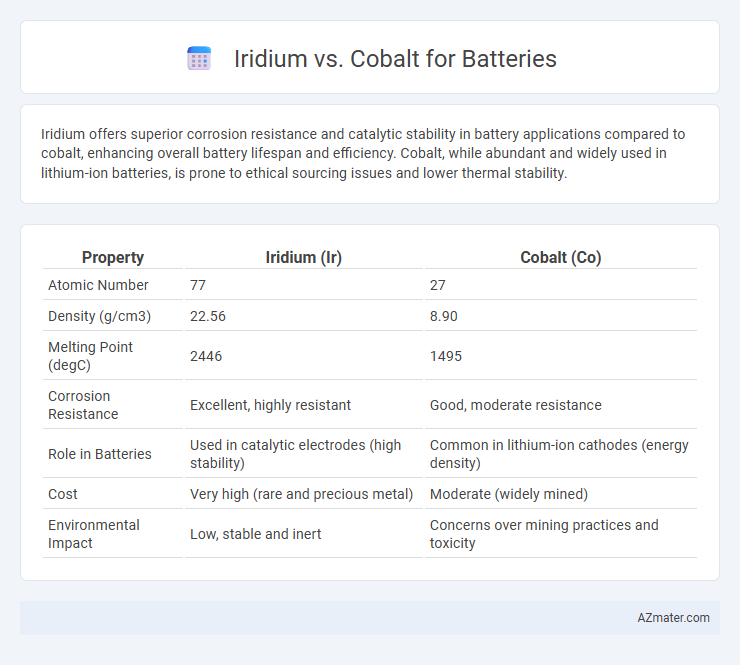
Iridium offers superior corrosion resistance and catalytic stability in battery applications compared to cobalt, enhancing overall battery lifespan and efficiency. Cobalt, while abundant and widely used in lithium-ion batteries, is prone to ethical sourcing issues and lower thermal stability.
Table of Comparison
| Property | Iridium (Ir) | Cobalt (Co) |
|---|---|---|
| Atomic Number | 77 | 27 |
| Density (g/cm3) | 22.56 | 8.90 |
| Melting Point (degC) | 2446 | 1495 |
| Corrosion Resistance | Excellent, highly resistant | Good, moderate resistance |
| Role in Batteries | Used in catalytic electrodes (high stability) | Common in lithium-ion cathodes (energy density) |
| Cost | Very high (rare and precious metal) | Moderate (widely mined) |
| Environmental Impact | Low, stable and inert | Concerns over mining practices and toxicity |
Introduction to Iridium and Cobalt in Battery Technology
Iridium and cobalt play distinct roles in battery technology, with iridium primarily used as a catalyst in certain advanced battery systems, enhancing oxygen evolution reactions and improving efficiency in electrolyzers and fuel cells. Cobalt is a critical component in lithium-ion batteries, serving as a key element in cathode materials like lithium cobalt oxide (LiCoO2), which provides high energy density and stability. The contrasting applications highlight iridium's importance in catalytic processes versus cobalt's role in energy storage and battery performance.
Chemical Properties Relevant to Batteries
Iridium exhibits exceptional corrosion resistance and catalytic activity, enhancing battery electrode durability and performance under harsh electrochemical conditions. Cobalt offers high electrical conductivity and stability in lithium-ion battery cathodes, promoting energy density and cycle life. The distinct chemical properties of iridium's noble metal status versus cobalt's transition metal reactivity impact their specific roles in battery technology optimization.
Abundance and Global Availability
Iridium is extremely rare, with a global abundance of about 0.001 parts per million in the Earth's crust, making it significantly less available than cobalt, which has an abundance of approximately 25 parts per million. Cobalt is more widely distributed, with major reserves found in the Democratic Republic of Congo, Russia, and Australia, supporting its large-scale use in battery production. Iridium's scarcity and limited supply chains pose challenges for cost-effective and sustainable battery manufacturing compared to the more abundant and globally accessible cobalt.
Energy Density and Performance Comparison
Iridium and cobalt play distinct roles in battery technology, with cobalt primarily used in lithium-ion cathodes to enhance energy density and cycle stability, achieving energy densities typically around 250 Wh/kg. Iridium, often used in specialized electrode applications, contributes to improved catalytic activity and battery longevity but does not directly increase energy density to the same extent as cobalt. Performance comparison highlights cobalt's dominance in energy storage capacity and commercial viability, while iridium's unique electrochemical properties support niche, high-performance applications requiring enhanced durability and charge efficiency.
Longevity and Cycle Life
Iridium and cobalt play crucial roles in battery longevity and cycle life, with cobalt commonly used in lithium-ion cathodes to enhance energy density and stability. Iridium, although rare and expensive, is primarily utilized in advanced battery designs such as solid oxide fuel cells, contributing to prolonged cycle life by improving electrode durability and corrosion resistance. Cobalt-rich cathodes typically deliver 500 to 1,000 charge-discharge cycles, while iridium-based electrodes can exceed these figures in specialized applications, supporting longer-lasting battery performance.
Environmental Impact and Sustainability
Iridium, used primarily as a catalyst in fuel cells, offers high durability and is highly recyclable but remains rare and expensive, limiting its large-scale environmental sustainability. Cobalt, critical in lithium-ion batteries for energy density and stability, faces significant environmental and ethical challenges due to toxic mining practices and supply chain concerns. Sustainable battery development demands reducing cobalt dependency through recycling and alternative materials, while iridium's scarcity necessitates efficient reuse to minimize ecological impact.
Cost Analysis: Iridium vs Cobalt
Iridium and cobalt both serve critical roles in battery technology but exhibit significant differences in cost implications. Cobalt, widely used in lithium-ion batteries, remains expensive due to limited supply and high demand, with prices typically around $30,000 per metric ton, while iridium's price exceeds $400,000 per kilogram, making it considerably costlier for large-scale battery applications. Cost analysis favors cobalt for commercial viability, but iridium's superior catalytic properties could justify expenses in specialized or high-performance battery systems.
Current Applications in Battery Industry
Iridium is predominantly utilized in advanced lithium-ion battery cathodes due to its high corrosion resistance and catalytic properties, enhancing battery lifespan and energy density. Cobalt remains a critical component in lithium cobalt oxide (LiCoO2) cathodes, widely used in consumer electronics and electric vehicles for its stability and high energy output. Emerging battery technologies are exploring reduced cobalt content to address cost and ethical concerns, while iridium's role is expanding in niche, high-performance battery applications.
Safety and Handling Considerations
Iridium offers superior chemical stability and corrosion resistance compared to cobalt, reducing risks related to battery thermal runaway and enhancing overall safety during operation. Cobalt-based batteries, while energy-dense, pose greater handling hazards due to their toxicity and potential for hazardous exposure in manufacturing and disposal processes. Effective safety protocols and advanced containment technologies are crucial when working with cobalt batteries to mitigate health risks and environmental impact.
Future Prospects and Research Directions
Iridium and cobalt are critical elements in battery technology, with cobalt currently dominating cathode materials for lithium-ion batteries due to its high energy density and stability. Future research aims to reduce cobalt dependency by exploring iridium's potential for enhancing battery lifespan and catalytic properties in solid-state batteries. Advances in material engineering and recycling methods are driving investigations into iridium-cobalt composites to improve battery performance and sustainability in next-generation energy storage systems.
Infographic: Iridium vs Cobalt for Battery
 azmater.com
azmater.com