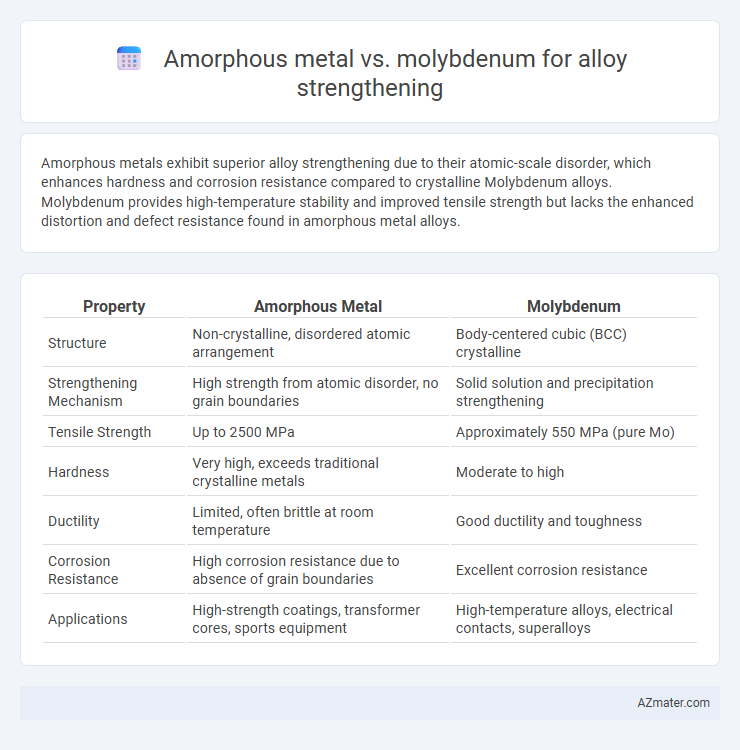
Amorphous metals exhibit superior alloy strengthening due to their atomic-scale disorder, which enhances hardness and corrosion resistance compared to crystalline Molybdenum alloys. Molybdenum provides high-temperature stability and improved tensile strength but lacks the enhanced distortion and defect resistance found in amorphous metal alloys.
Table of Comparison
| Property | Amorphous Metal | Molybdenum |
|---|---|---|
| Structure | Non-crystalline, disordered atomic arrangement | Body-centered cubic (BCC) crystalline |
| Strengthening Mechanism | High strength from atomic disorder, no grain boundaries | Solid solution and precipitation strengthening |
| Tensile Strength | Up to 2500 MPa | Approximately 550 MPa (pure Mo) |
| Hardness | Very high, exceeds traditional crystalline metals | Moderate to high |
| Ductility | Limited, often brittle at room temperature | Good ductility and toughness |
| Corrosion Resistance | High corrosion resistance due to absence of grain boundaries | Excellent corrosion resistance |
| Applications | High-strength coatings, transformer cores, sports equipment | High-temperature alloys, electrical contacts, superalloys |
Introduction to Alloy Strengthening
Amorphous metals, characterized by their disordered atomic structure, offer superior strength and hardness compared to traditional crystalline metals, making them effective for alloy strengthening through enhanced resistance to deformation. Molybdenum, a refractory metal with a high melting point and significant solid solution strengthening capabilities, improves alloy strength by increasing creep resistance and maintaining structural stability at elevated temperatures. Both materials contribute uniquely to alloy strengthening: amorphous metals enhance toughness and strength via structural disorder, while molybdenum provides thermal stability and improved mechanical properties through atomic-scale reinforcement.
Overview of Amorphous Metals
Amorphous metals, also known as metallic glasses, are non-crystalline alloys exhibiting a disordered atomic structure that enhances strength and hardness compared to traditional crystalline metals like molybdenum. These materials offer superior corrosion resistance and high elastic limits due to the absence of grain boundaries, making them ideal for advanced alloy strengthening applications. Unlike molybdenum, which contributes strength primarily through solid solution and precipitation hardening, amorphous metals rely on their unique atomic arrangement to provide exceptional mechanical properties and wear resistance.
Properties of Molybdenum as an Alloying Element
Molybdenum significantly enhances alloy strength due to its high melting point, excellent corrosion resistance, and ability to improve hardenability and creep resistance in steel. Unlike amorphous metals, molybdenum contributes to grain refinement and solid solution strengthening, which increases tensile strength and wear resistance in high-performance alloys. Its role as a refractory metal makes it essential for strengthening alloys used in extreme environments such as aerospace and nuclear reactors.
Comparative Mechanical Strength
Amorphous metals exhibit superior mechanical strength due to their non-crystalline atomic structure, providing high yield strength and hardness compared to crystalline molybdenum-based alloys, which rely on their stable crystal lattice for strength. Molybdenum alloys offer excellent high-temperature strength and creep resistance, but their mechanical properties are often limited by grain boundary weaknesses and brittleness. The amorphous structure enables enhanced elastic strain limit and toughness, while molybdenum alloys benefit from established metallurgical processes that optimize load-bearing capacity and thermal stability.
Corrosion Resistance: Amorphous Metal vs Molybdenum
Amorphous metals exhibit superior corrosion resistance compared to molybdenum due to their non-crystalline atomic structure, which eliminates grain boundaries that typically serve as corrosion initiation sites. Molybdenum, while known for enhancing corrosion resistance in crystalline alloys, is susceptible to localized corrosion such as pitting under aggressive environments. Therefore, amorphous metals provide enhanced durability in harsh chemical conditions, outperforming molybdenum-containing alloys in resisting corrosion degradation.
Thermal Stability and Performance
Amorphous metals exhibit superior thermal stability compared to molybdenum, maintaining their structural integrity at elevated temperatures without crystallization, which enhances alloy performance under high-heat conditions. Molybdenum, while effective in strengthening alloys through solid solution and precipitation hardening, can experience grain growth and reduced stability during thermal exposure. The enhanced thermal stability of amorphous metals contributes to prolonged mechanical strength and resistance to deformation, making them advantageous for high-performance alloy applications.
Manufacturing Techniques and Process Compatibility
Amorphous metals are typically produced through rapid solidification techniques such as melt spinning or high-pressure die casting, which enable their non-crystalline atomic structure, whereas molybdenum is incorporated into alloys primarily via conventional powder metallurgy or melting and casting processes due to its high melting point and density. Manufacturing amorphous metals requires precise cooling rates exceeding 10^5 K/s to prevent crystallization, limiting process scalability but offering superior strength and corrosion resistance, while molybdenum's compatibility with standard alloy processing enhances its integration for strength and high-temperature stability. The distinct thermal and mechanical properties of amorphous metals demand specialized handling during manufacturing, contrasting with molybdenum's established process adaptability in steel and superalloy production.
Cost Analysis and Material Availability
Amorphous metals, also known as metallic glasses, offer superior strength and corrosion resistance compared to conventional alloys but tend to have higher production costs due to complex manufacturing processes and limited large-scale availability. Molybdenum, widely used as an alloying element for strengthening, is more cost-effective and readily available globally, benefiting from established mining and refining industries. Cost analysis highlights molybdenum's advantage for large-volume applications, whereas amorphous metals are better suited for high-performance, niche applications where material properties justify the expense.
Applications in Industry: Where They Excel
Amorphous metals exhibit exceptional strength and corrosion resistance, making them ideal for high-performance sporting goods, military armor, and precision medical instruments where durability and reliability are critical. Molybdenum enhances alloy strength through its high melting point and resistance to wear, commonly used in aerospace components, power generation turbines, and industrial machinery for improved heat tolerance and structural integrity. Both materials excel in applications demanding superior mechanical properties, but amorphous metals are preferred for lightweight, wear-resistant parts while molybdenum alloys are favored in high-temperature and high-stress environments.
Future Trends in Alloy Strengthening Technologies
Amorphous metals exhibit superior strength and corrosion resistance due to their disordered atomic structure, making them promising candidates for next-generation lightweight alloys. Molybdenum enhances alloy strength through solid-solution strengthening and carbide formation, particularly in high-temperature applications like aerospace and power plants. Future trends emphasize hybrid alloys combining amorphous metal matrices with molybdenum additives to achieve unprecedented mechanical performance and thermal stability.
Infographic: Amorphous metal vs Molybdenum for Alloy strengthening
 azmater.com
azmater.com