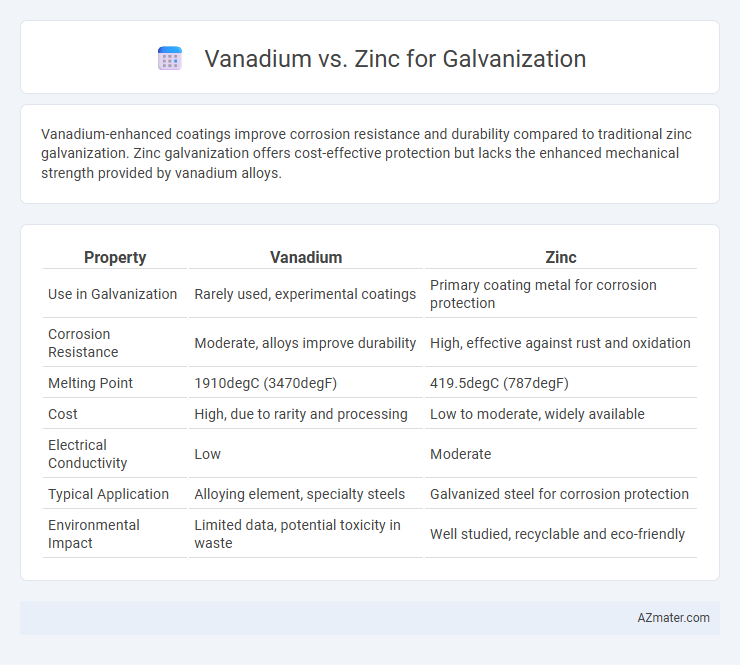
Vanadium-enhanced coatings improve corrosion resistance and durability compared to traditional zinc galvanization. Zinc galvanization offers cost-effective protection but lacks the enhanced mechanical strength provided by vanadium alloys.
Table of Comparison
| Property | Vanadium | Zinc |
|---|---|---|
| Use in Galvanization | Rarely used, experimental coatings | Primary coating metal for corrosion protection |
| Corrosion Resistance | Moderate, alloys improve durability | High, effective against rust and oxidation |
| Melting Point | 1910degC (3470degF) | 419.5degC (787degF) |
| Cost | High, due to rarity and processing | Low to moderate, widely available |
| Electrical Conductivity | Low | Moderate |
| Typical Application | Alloying element, specialty steels | Galvanized steel for corrosion protection |
| Environmental Impact | Limited data, potential toxicity in waste | Well studied, recyclable and eco-friendly |
Introduction: The Importance of Galvanization
Galvanization is crucial for protecting steel and iron structures from corrosion, significantly extending their lifespan and reducing maintenance costs. Zinc is widely used as the primary coating metal due to its excellent sacrificial protection and cost-effectiveness. Vanadium, although less common, has been explored for its potential to enhance coating hardness and corrosion resistance in specialized applications.
Overview of Vanadium and Zinc in Metallurgy
Vanadium is a transition metal valued in metallurgy for its ability to increase steel hardness, strength, and corrosion resistance, often used in alloying to enhance mechanical properties. Zinc, primarily applied in galvanization, serves as a sacrificial anode to protect steel and iron surfaces from rust and corrosion through a process of electrochemical passivation. Both elements play critical roles in metal treatment, with vanadium enhancing internal structural qualities and zinc providing external protective coatings for longevity.
Chemical Properties: Vanadium vs Zinc
Vanadium exhibits a high oxidation resistance and forms stable oxide layers, enhancing corrosion protection in galvanization, whereas zinc acts as a sacrificial anode due to its lower electrochemical potential. Zinc's galvanic behavior provides effective rust prevention by preferentially corroding and protecting the base metal, while vanadium contributes to hardness and wear resistance in alloy-coated surfaces. The chemical stability of vanadium oxide under various environmental conditions contrasts with zinc's more reactive zinc oxide, influencing their respective durability and protective longevity in galvanized coatings.
Galvanization Process Using Zinc
The galvanization process using zinc involves dipping steel or iron into molten zinc to create a protective, corrosion-resistant coating that prevents rust. Zinc acts as a sacrificial anode, corroding preferentially and thereby protecting the base metal beneath it. Unlike vanadium, zinc is widely favored in galvanization due to its excellent adhesion properties, cost-effectiveness, and well-established industrial use in hot-dip and electrogalvanization methods.
Potential Application of Vanadium in Galvanization
Vanadium offers promising potential for galvanization by enhancing corrosion resistance and improving the mechanical properties of steel coatings compared to traditional zinc galvanization. Its ability to form a dense, adherent oxide layer provides superior protection against rust and extends the lifespan of galvanized materials, especially in harsh industrial environments. Research indicates that integrating vanadium into galvanization processes can lead to more durable coatings, reducing maintenance costs and increasing structural integrity in automotive and construction industries.
Corrosion Resistance: Comparative Analysis
Vanadium-enhanced coatings exhibit superior corrosion resistance compared to traditional zinc galvanization due to the formation of more stable and dense oxide layers that inhibit rust development. Zinc provides effective sacrificial protection by corroding preferentially, but vanadium alloys improve longevity by enhancing passivation and reducing coating degradation in harsh environments. Studies show that vanadium's microstructural properties significantly extend the lifespan of galvanized steel in marine and industrial applications.
Cost and Availability of Vanadium and Zinc
Vanadium is typically more expensive and less readily available compared to zinc, which is abundant and cost-effective for galvanization purposes. Zinc's widespread availability from mining operations and established supply chains drives lower prices, making it the preferred choice for large-scale corrosion protection. The higher cost and limited supply of vanadium restrict its use mainly to specialized applications rather than mainstream galvanization.
Environmental Impact and Sustainability
Vanadium and zinc both serve as protective coatings in galvanization, but zinc remains the preferred choice due to its lower environmental footprint and greater recyclability. Zinc's natural abundance and ability to form compact, self-healing layers reduce corrosion without toxic byproducts, promoting sustainability in steel protection. Vanadium, while offering enhanced strength, involves more energy-intensive extraction and processing, leading to higher carbon emissions and less eco-friendly galvanization practices.
Industrial Applications and Case Studies
Vanadium-enhanced coatings improve corrosion resistance and mechanical strength in industrial galvanization, outperforming traditional zinc layers in harsh environments like marine and chemical plants. Case studies highlight vanadium's ability to extend the lifespan of steel structures by up to 30%, reducing maintenance costs and downtime. Zinc remains widely used for cost-effective protection but lacks the enhanced durability and wear resistance provided by vanadium alloys in high-stress applications.
Future Trends in Galvanization Technologies
Vanadium is emerging as a promising alloying element in galvanization due to its ability to enhance coating adhesion and corrosion resistance, outperforming traditional zinc-only coatings. Future trends highlight the integration of vanadium-zinc hybrid coatings that leverage vanadium's strength and zinc's sacrificial properties for longer-lasting protection in harsh environments. Advances in nanotechnology and alloy composition optimization aim to produce more efficient, eco-friendly galvanization processes that prioritize durability and reduced environmental impact.
Infographic: Vanadium vs Zinc for Galvanization
 azmater.com
azmater.com