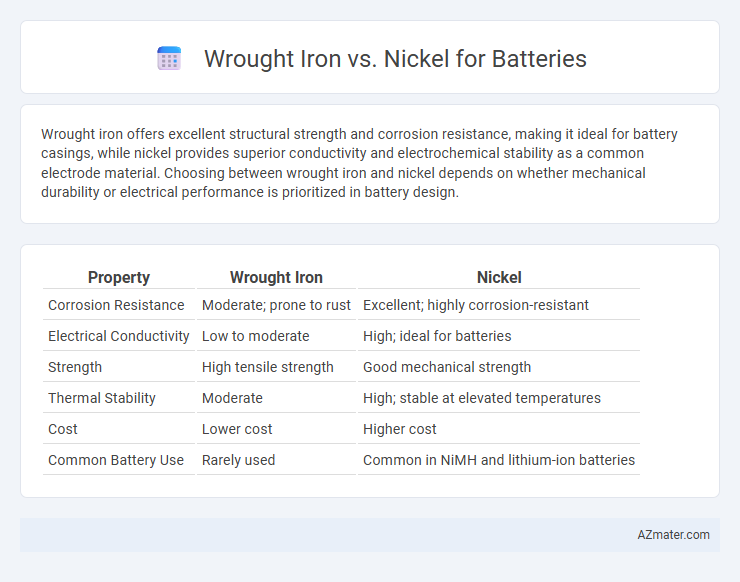
Wrought iron offers excellent structural strength and corrosion resistance, making it ideal for battery casings, while nickel provides superior conductivity and electrochemical stability as a common electrode material. Choosing between wrought iron and nickel depends on whether mechanical durability or electrical performance is prioritized in battery design.
Table of Comparison
| Property | Wrought Iron | Nickel |
|---|---|---|
| Corrosion Resistance | Moderate; prone to rust | Excellent; highly corrosion-resistant |
| Electrical Conductivity | Low to moderate | High; ideal for batteries |
| Strength | High tensile strength | Good mechanical strength |
| Thermal Stability | Moderate | High; stable at elevated temperatures |
| Cost | Lower cost | Higher cost |
| Common Battery Use | Rarely used | Common in NiMH and lithium-ion batteries |
Introduction to Wrought Iron and Nickel in Batteries
Wrought iron is rarely used in batteries due to its lower conductivity and susceptibility to corrosion compared to other metals. Nickel, specifically nickel-based compounds like nickel-metal hydride (NiMH), plays a crucial role in battery technology for its high energy density, durability, and resistance to oxidation. The choice between wrought iron and nickel in batteries highlights the superior electrochemical properties of nickel, making it a preferred material for rechargeable battery electrodes.
Material Properties: Wrought Iron vs Nickel
Wrought iron exhibits excellent ductility and corrosion resistance but lacks the high electrical conductivity and electrochemical stability found in nickel, making it less suitable for battery electrodes. Nickel's superior conductivity and resistance to oxidation enable better charge transfer efficiency and longer battery life, essential for high-performance applications. The choice between wrought iron and nickel heavily depends on the battery type, with nickel often preferred in lithium-ion and alkaline cells due to its favorable material properties.
Electrochemical Performance Comparison
Wrought iron exhibits lower electrochemical stability and higher corrosion rates compared to nickel, limiting its efficiency and lifespan in battery applications. Nickel demonstrates superior charge capacity, better cycling stability, and enhanced resistance to oxidation, making it more suitable for high-performance batteries. The electrochemical impedance of nickel-based electrodes is significantly lower, contributing to improved conductivity and faster charge-discharge rates.
Cost Analysis: Wrought Iron vs Nickel
Wrought iron offers a lower initial material cost compared to nickel, making it a budget-friendly option for battery components with basic conductivity requirements. Nickel, despite its higher price per kilogram, provides superior corrosion resistance and electrical efficiency, potentially reducing long-term maintenance and replacement expenses. Evaluating the total cost of ownership for batteries involves balancing the upfront affordability of wrought iron against the durability and performance benefits provided by nickel.
Environmental Impact and Sustainability
Wrought iron batteries exhibit lower toxicity and higher recyclability, reducing environmental hazards compared to nickel-based batteries, which often involve toxic mining processes and challenging disposal methods. Nickel extraction and refining contribute significantly to greenhouse gas emissions and resource depletion, while wrought iron's abundant availability supports sustainable material sourcing. Lifecycle analyses reveal wrought iron batteries offer enhanced environmental performance and improved sustainability through easier recycling and reduced ecological footprint.
Lifespan and Durability in Battery Applications
Wrought iron, characterized by its high tensile strength and resistance to corrosion, offers moderate durability in battery casings but tends to have a shorter lifespan due to its susceptibility to oxidative degradation under prolonged electrochemical conditions. Nickel exhibits superior lifespan and durability in battery applications because of its excellent corrosion resistance, high melting point, and ability to maintain structural integrity during repeated charge-discharge cycles. Consequently, nickel is preferred over wrought iron for critical battery components, enhancing overall battery longevity and reliability.
Safety Considerations of Wrought Iron and Nickel
Wrought iron exhibits high corrosion resistance and structural integrity, reducing the risk of leakage or short circuits in battery applications. Nickel provides excellent chemical stability and resistance to oxidation, enhancing safety by maintaining consistent electrical conductivity under varying temperatures. Both materials contribute to battery safety through durability, but nickel's superior resistance to high-temperature degradation makes it more suitable for demanding thermal conditions.
Advancements in Wrought Iron-Based Batteries
Wrought iron-based batteries have seen significant advancements due to their enhanced electrochemical stability and higher corrosion resistance compared to nickel counterparts, leading to improved cycle life and energy density. Innovations in nanoscale wrought iron electrode design have increased charge transfer efficiency and reduced dendrite formation. These improvements position wrought iron as a promising, cost-effective alternative in battery technology, especially for sustainable energy storage applications.
Applications and Market Trends
Wrought iron exhibits limited use in battery applications due to its lower conductivity and corrosion resistance compared to nickel, which remains a preferred material in battery electrodes and casings for lithium-ion and nickel-metal hydride batteries. Nickel's superior electrochemical properties support efficient energy storage and discharge cycles, driving its dominance in electric vehicle (EV) batteries and renewable energy storage systems. Market trends highlight growing demand for nickel driven by EV adoption and grid storage needs, while wrought iron finds niche roles mainly in structural components outside battery technology.
Future Prospects for Wrought Iron and Nickel in Energy Storage
Wrought iron shows limited potential in future battery technologies due to its lower conductivity and corrosion resistance compared to nickel, which remains a critical material in advanced energy storage applications. Nickel's high energy density, stability, and catalytic properties make it essential for next-generation lithium-ion and solid-state batteries, driving ongoing research into nickel-based cathodes and alloys. Innovations in nickel recycling and extraction are expected to enhance sustainability and cost-efficiency, securing its dominant role in the evolving energy storage market.
Infographic: Wrought iron vs Nickel for Battery
 azmater.com
azmater.com