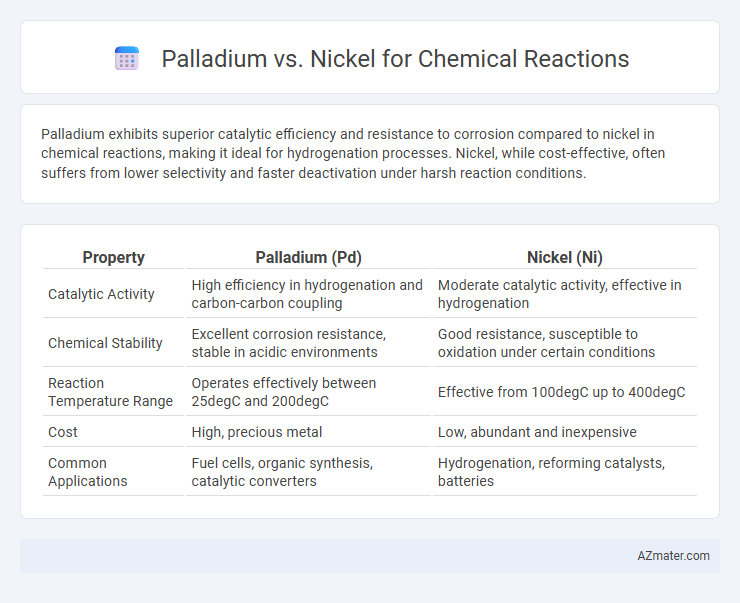
Palladium exhibits superior catalytic efficiency and resistance to corrosion compared to nickel in chemical reactions, making it ideal for hydrogenation processes. Nickel, while cost-effective, often suffers from lower selectivity and faster deactivation under harsh reaction conditions.
Table of Comparison
| Property | Palladium (Pd) | Nickel (Ni) |
|---|---|---|
| Catalytic Activity | High efficiency in hydrogenation and carbon-carbon coupling | Moderate catalytic activity, effective in hydrogenation |
| Chemical Stability | Excellent corrosion resistance, stable in acidic environments | Good resistance, susceptible to oxidation under certain conditions |
| Reaction Temperature Range | Operates effectively between 25degC and 200degC | Effective from 100degC up to 400degC |
| Cost | High, precious metal | Low, abundant and inexpensive |
| Common Applications | Fuel cells, organic synthesis, catalytic converters | Hydrogenation, reforming catalysts, batteries |
Introduction to Palladium and Nickel in Chemical Reactions
Palladium and nickel are transition metals widely used as catalysts in chemical reactions due to their unique electronic configurations and surface properties. Palladium exhibits exceptional catalytic activity in hydrogenation and carbon-carbon coupling reactions, often facilitating high selectivity under mild conditions. Nickel serves as a cost-effective alternative, catalyzing various hydrogenation and reforming processes, though with generally lower selectivity compared to palladium.
Chemical Properties: Palladium vs Nickel
Palladium exhibits superior catalytic activity in hydrogenation and carbon-carbon coupling reactions due to its ability to adsorb hydrogen and activate organic molecules efficiently compared to nickel. Nickel, although less expensive, demonstrates higher resistance to poisoning by sulfur compounds but generally requires higher temperatures to achieve similar reaction rates. The chemical properties of palladium, including its variable oxidation states (Pd0, Pd2+), enhance its versatility in catalysis, whereas nickel primarily functions in the Ni0 state with more limited redox flexibility.
Catalytic Efficiency Comparison
Palladium exhibits superior catalytic efficiency compared to nickel in facilitating hydrogenation and carbon-carbon coupling reactions due to its higher activity and selectivity under mild conditions. Nickel, while cost-effective and effective for hydrogenation, often requires higher temperatures and pressures to achieve comparable reaction rates, limiting its application in sensitive synthetic processes. The distinct electronic properties of palladium allow for more efficient adsorption and activation of reactants, enhancing turnover frequencies and overall catalytic performance.
Cost and Availability of Palladium and Nickel
Nickel offers a cost-effective alternative to palladium in chemical reactions due to its abundant availability and lower market price, making it suitable for large-scale industrial applications. Palladium, though more expensive and less abundant, provides superior catalytic efficiency and selectivity in specific reactions such as hydrogenation and carbon-carbon coupling. The choice between palladium and nickel depends on balancing the initial catalyst cost with the desired reaction performance and scalability.
Environmental Impact and Sustainability
Palladium catalysts enable efficient chemical reactions with high selectivity and often require lower energy input, resulting in reduced greenhouse gas emissions compared to nickel-based catalysts. Nickel, though abundant and cost-effective, poses environmental risks due to its potential toxicity and less efficient catalytic performance, leading to higher waste generation and energy consumption. Sustainable chemical processes increasingly favor palladium for its recyclability and lower ecological footprint, despite the higher initial cost and resource scarcity.
Selectivity and Yield Differences
Palladium catalysts exhibit higher selectivity in chemical reactions due to their unique electronic configuration, which facilitates precise bond activation and reduces side reactions. Nickel catalysts often provide higher yields in hydrogenation processes owing to their robust activity and cost-effectiveness, though they may produce more by-products compared to palladium. Selectivity differences stem from palladium's ability to favor specific reaction pathways, while nickel's broader reactivity profile influences overall yield and product distribution.
Industrial Applications: Case Studies
Palladium and nickel are extensively used catalysts in industrial chemical reactions, with palladium excelling in selective hydrogenation and carbon-carbon coupling due to its superior activity and resistance to poisoning. Nickel is favored in large-scale processes like hydrocracking and steam reforming because of its cost-effectiveness and high catalytic efficiency in hydrogenation reactions involving hydrocarbons. Case studies in the pharmaceutical industry demonstrate palladium's critical role in Suzuki and Heck cross-coupling reactions for complex molecule synthesis, while nickel catalysts are predominant in petrochemical refining for converting heavy oils into lighter fractions.
Palladium vs Nickel: Reaction Mechanism Insights
Palladium catalyzes chemical reactions through facile oxidative addition and reductive elimination steps, often enabling more efficient cross-coupling reactions compared to nickel. Nickel tends to engage in single-electron transfer mechanisms, allowing radical intermediates and unique reactivity under milder conditions. The electronic structure differences between palladium and nickel result in distinct catalytic cycles that influence selectivity, turnover frequency, and substrate scope in various organic transformations.
Handling and Safety Considerations
Palladium catalysts exhibit superior chemical stability and lower toxicity compared to nickel, making them safer for handling in laboratory and industrial settings. Nickel compounds can pose significant risks due to their potential carcinogenicity and skin sensitization, requiring rigorous protective measures such as gloves and fume hoods. Proper ventilation and avoidance of inhalation or prolonged skin contact are critical when working with nickel catalysts to mitigate health hazards and ensure safe chemical reactions.
Choosing the Right Catalyst for Your Reaction
Selecting the right catalyst between palladium and nickel depends on the specific chemical reaction and its conditions. Palladium excels in cross-coupling reactions like Suzuki and Heck due to its high activity and selectivity, while nickel offers cost-effective alternatives for hydrogenation and reductive couplings with broader functional group tolerance. Consider reaction scale, substrate sensitivity, and desired turnover frequencies to optimize efficiency and product yield effectively.
Infographic: Palladium vs Nickel for Chemical Reaction
 azmater.com
azmater.com