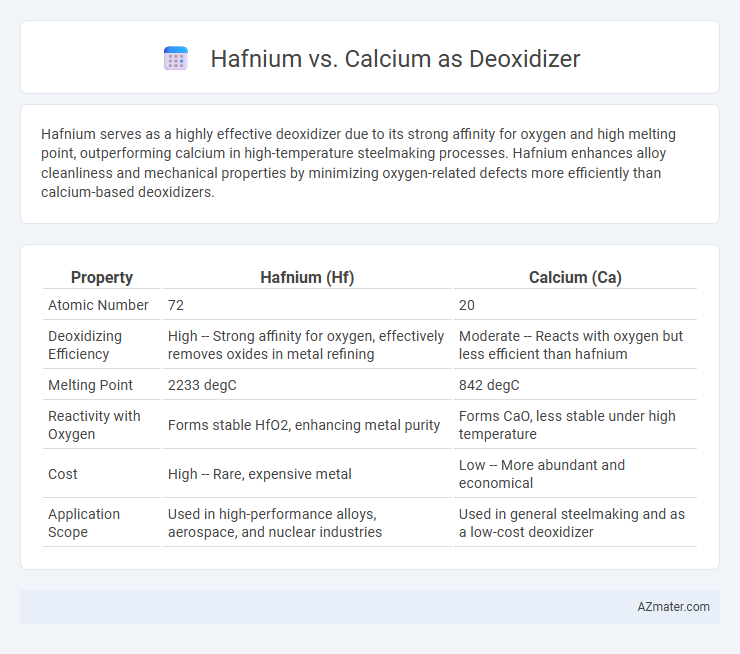
Hafnium serves as a highly effective deoxidizer due to its strong affinity for oxygen and high melting point, outperforming calcium in high-temperature steelmaking processes. Hafnium enhances alloy cleanliness and mechanical properties by minimizing oxygen-related defects more efficiently than calcium-based deoxidizers.
Table of Comparison
| Property | Hafnium (Hf) | Calcium (Ca) |
|---|---|---|
| Atomic Number | 72 | 20 |
| Deoxidizing Efficiency | High -- Strong affinity for oxygen, effectively removes oxides in metal refining | Moderate -- Reacts with oxygen but less efficient than hafnium |
| Melting Point | 2233 degC | 842 degC |
| Reactivity with Oxygen | Forms stable HfO2, enhancing metal purity | Forms CaO, less stable under high temperature |
| Cost | High -- Rare, expensive metal | Low -- More abundant and economical |
| Application Scope | Used in high-performance alloys, aerospace, and nuclear industries | Used in general steelmaking and as a low-cost deoxidizer |
Introduction to Deoxidizers: Purpose and Importance
Hafnium and calcium play crucial roles as deoxidizers in metal refining, effectively removing oxygen to prevent oxidation and enhance metal quality. Hafnium's high melting point and strong affinity for oxygen make it ideal for deoxidizing high-performance alloys used in aerospace and nuclear industries. Calcium, known for its cost-effectiveness and efficient oxygen removal, is widely used in steelmaking to improve strength and ductility by reducing oxidized impurities.
Overview of Hafnium as a Deoxidizer
Hafnium serves as an effective deoxidizer in metallurgical processes due to its strong affinity for oxygen, forming stable oxides that improve metal purity and mechanical properties. Compared to calcium, hafnium provides superior high-temperature oxidation resistance and enhanced grain refinement in alloys. Its application is particularly valuable in high-performance steel and superalloys, where precise control of oxygen levels is critical for material strength and durability.
Calcium’s Role in Deoxidation Processes
Calcium serves as an effective deoxidizer in metallurgical processes due to its strong affinity for oxygen, forming stable calcium oxide (CaO) compounds that efficiently remove dissolved oxygen from molten metals. Unlike hafnium, which is less commonly used for deoxidation, calcium's lower cost and high reactivity make it a preferred choice in steelmaking to enhance metal cleanliness and improve mechanical properties. Its ability to modify inclusion morphology and reduce oxides contributes significantly to the overall quality and performance of alloys.
Chemical Properties: Hafnium vs Calcium
Hafnium exhibits superior chemical stability and a higher melting point (2233 degC) compared to calcium (842 degC), making it more effective in high-temperature deoxidation processes. The strong affinity of hafnium for oxygen forms stable hafnium oxides, which efficiently remove oxygen from molten metals, whereas calcium, with its lower electronegativity and reactivity, offers faster but less stable oxide formation. Hafnium's resistance to oxidation and its ability to form dense oxide layers confer advantages in controlling oxygen content and improving the overall quality of metal alloys.
Efficiency Comparison in Removing Oxygen
Hafnium exhibits superior efficiency over calcium as a deoxidizer due to its higher affinity for oxygen and greater stability in forming oxides, leading to more effective oxygen removal in metallurgical processes. Its oxide, hafnium dioxide (HfO2), has a high melting point and strong chemical stability, which enhances the purification of molten metals by reducing oxygen content more effectively. In contrast, calcium forms calcium oxide (CaO), which is less thermodynamically stable, making hafnium a preferable choice for applications requiring intense deoxidation and improved metal quality.
Impact on Alloy Quality and Characteristics
Hafnium as a deoxidizer significantly enhances alloy quality by improving high-temperature strength and corrosion resistance, while calcium mainly reduces oxide inclusions, promoting cleaner steel with improved toughness. Hafnium forms stable oxides that refine grain structure and increase hardness, whereas calcium modifies non-metallic inclusions, aiding in better machinability and ductility. The choice between hafnium and calcium directly impacts the final mechanical properties and performance characteristics of the alloy, making hafnium preferable for high-performance applications requiring superior heat and corrosion resistance.
Cost Analysis: Hafnium vs Calcium
Hafnium, as a deoxidizer, exhibits superior performance but comes with a significantly higher cost compared to calcium, primarily due to its rarity and complex extraction process. Calcium remains a more economically viable option in large-scale steel production, offering effective deoxidation at a fraction of hafnium's price per kilogram. Cost analysis reveals that while hafnium may reduce impurities more efficiently, the overall expense often outweighs the benefits for most commercial metallurgical applications.
Industrial Applications and Use Cases
Hafnium and calcium serve as effective deoxidizers in metallurgical processes, with hafnium favored for high-performance alloys due to its high melting point and excellent oxidation resistance, enhancing aerospace and nuclear reactor component durability. Calcium is widely used in steelmaking to improve sulfur inclusions and slag quality, optimizing manufacturing efficiency and product quality in construction and automotive industries. Industrial applications of hafnium focus on superalloys and corrosion-resistant materials, while calcium is primarily utilized for cost-effective impurity control in large-scale steel production.
Environmental and Safety Considerations
Hafnium offers superior corrosion resistance and stability compared to calcium when used as a deoxidizer, reducing hazardous byproducts and environmental contamination. Calcium, while effective and more cost-efficient, poses higher risks of oxidation and reactivity that require stringent handling protocols to prevent fires and toxic emissions. The lower toxicity and environmental footprint of hafnium make it a safer choice for sustainable metallurgical applications.
Conclusion: Choosing the Right Deoxidizer
Hafnium offers superior high-temperature stability and better resistance to oxidation compared to calcium, making it ideal for advanced metallurgical processes where performance under extreme conditions is critical. Calcium, while more cost-effective and abundant, excels in applications requiring effective oxygen removal at moderate temperatures, providing efficient deoxidation with simpler handling. Selecting the right deoxidizer depends on balancing material properties, cost, and process requirements, with hafnium preferred for high-performance alloys and calcium suited for standard steelmaking.
Infographic: Hafnium vs Calcium for Deoxidizer
 azmater.com
azmater.com