Bronze coins offer greater durability and corrosion resistance compared to iron coins, which are prone to rust and wear over time. The higher copper content in bronze also provides a distinct, attractive patina, enhancing the coin's aesthetic and numismatic value.
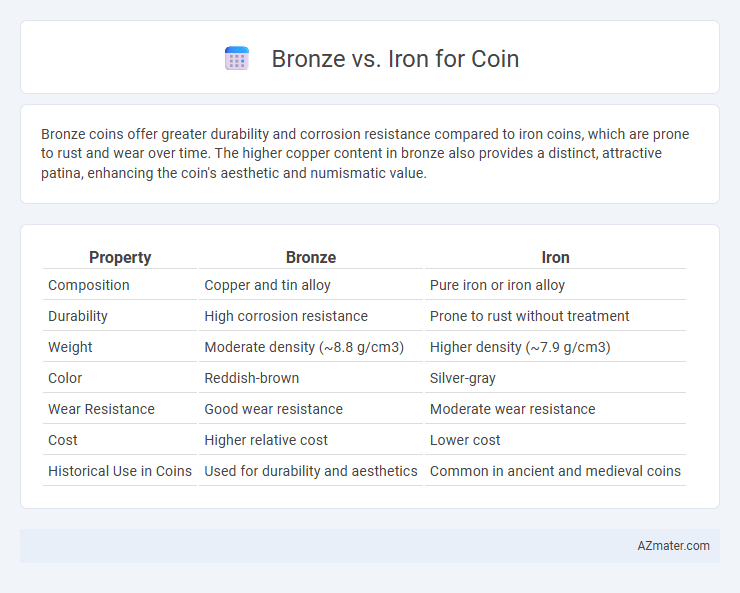
Table of Comparison
| Property | Bronze | Iron |
|---|---|---|
| Composition | Copper and tin alloy | Pure iron or iron alloy |
| Durability | High corrosion resistance | Prone to rust without treatment |
| Weight | Moderate density (~8.8 g/cm3) | Higher density (~7.9 g/cm3) |
| Color | Reddish-brown | Silver-gray |
| Wear Resistance | Good wear resistance | Moderate wear resistance |
| Cost | Higher relative cost | Lower cost |
| Historical Use in Coins | Used for durability and aesthetics | Common in ancient and medieval coins |
Introduction to Coinage Metals: Bronze vs Iron
Bronze, an alloy primarily of copper and tin, has historically been favored for coinage due to its durability, resistance to corrosion, and appealing color, making it ideal for long-lasting currency. Iron, while abundant and inexpensive, tends to corrode quickly and wear down, limiting its effectiveness and longevity as a coinage metal. The metal properties of bronze ensure better preservation and aesthetic qualities, which contributed to its preference over iron in early coin minting.
Historical Use of Bronze and Iron in Coin Making
Bronze, an alloy primarily of copper and tin, was historically favored in coin making due to its durability, corrosion resistance, and ease of casting, especially in ancient Greek and Roman civilizations. Iron, being more prone to rust and harder to work with, saw limited use in coins but was occasionally employed in periods of metal scarcity or for low-denomination currency in medieval Europe and Asia. The transition from bronze to iron coins often reflected economic shifts and availability of resources rather than improved metallurgical properties.
Composition and Properties: Bronze vs Iron
Bronze coins consist primarily of copper alloyed with tin, offering high corrosion resistance and durability, making them suitable for long-term circulation. Iron coins, composed mainly of iron with minimal impurities, are prone to oxidation and rust, which affects their longevity and aesthetic appeal. The superior hardness and patina formation of bronze enhance coin preservation, while iron's magnetic properties and brittleness limit its effectiveness in coinage.
Durability and Longevity: Which Metal Lasts Longer?
Bronze coins exhibit superior durability due to their higher resistance to corrosion and wear, making them ideal for long-term preservation. Iron coins, while initially strong, are prone to rust and degradation when exposed to moisture, significantly reducing their longevity. Therefore, bronze offers a longer lifespan and better maintains its structural integrity over time compared to iron.
Corrosion Resistance: Bronze Compared to Iron
Bronze offers superior corrosion resistance compared to iron, making it an ideal material for coins exposed to varying environmental conditions. The copper content in bronze forms a protective patina that prevents further oxidation, whereas iron is prone to rust and deterioration when exposed to moisture and air. This enhanced durability ensures bronze coins maintain their appearance and structural integrity over extended periods.
Cost and Availability of Bronze and Iron for Coins
Bronze, an alloy primarily composed of copper and tin, is costlier than iron due to the scarcity and higher extraction expenses of copper and tin compared to iron ore. Iron is more abundant and widely available, making it a cheaper material choice for minting coins. However, bronze coins generally offer better corrosion resistance and a more refined appearance, influencing cost considerations beyond just raw material availability.
Aesthetic Appeal: Appearance of Bronze vs Iron Coins
Bronze coins exhibit a warm, golden-brown hue with a smooth patina that enhances their visual appeal over time, making them highly favored by collectors. Iron coins typically display a darker, matte grey or blackened surface prone to rust and corrosion, which can diminish their aesthetic quality. The natural aging process of bronze creates attractive verdigris tones, whereas iron's susceptibility to oxidation often results in an uneven, less visually pleasing finish.
Minting Process: Working with Bronze vs Iron
Minting coins from bronze involves a more straightforward casting and striking process due to bronze's lower melting point of approximately 950degC and its excellent malleability, allowing for finer detail in coin designs. Iron, with a higher melting point around 1538degC and increased hardness, requires more advanced equipment and higher temperatures for melting and forging, making the minting process more labor-intensive and less precise in capturing intricate details. The oxidation of iron during minting also necessitates additional surface treatment steps to prevent rust, unlike bronze, which has natural corrosion resistance from its copper-tin alloy composition.
Collectibility and Value: Bronze Coins vs Iron Coins
Bronze coins generally hold higher collectibility and value than iron coins due to their durability, corrosion resistance, and historical significance in ancient currency systems. Iron coins are more prone to rust and deterioration, which negatively impacts their preservation and market demand among collectors. Limited availability of well-preserved bronze coins further enhances their rarity and appeal in numismatic collections.
Conclusion: Choosing Between Bronze and Iron for Coins
Bronze coins offer superior durability, corrosion resistance, and a classic aesthetic compared to iron, making them ideal for long-term circulation and collector appeal. Iron coins tend to rust quickly and wear down faster, which can diminish their usability and visual quality over time. Selecting bronze over iron ensures better preservation of details and lasting value in numismatic and everyday uses.
Infographic: Bronze vs Iron for Coin
 azmater.com
azmater.com