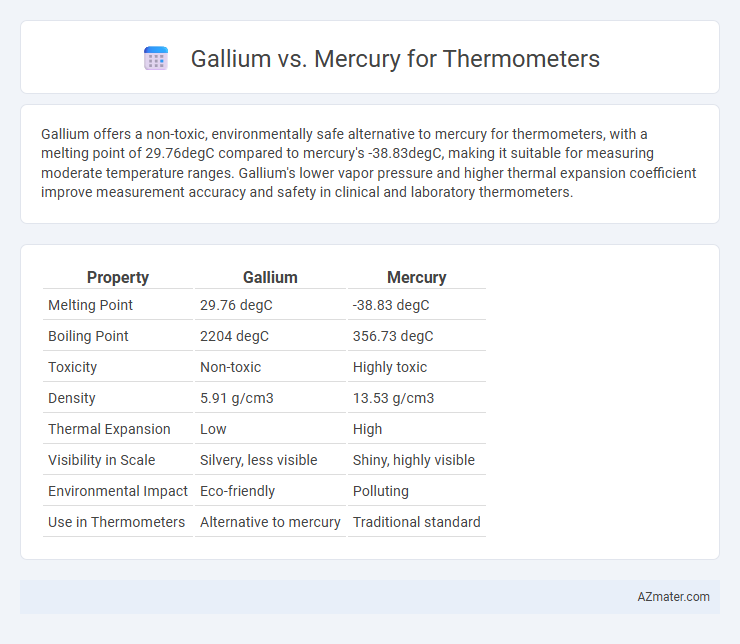
Gallium offers a non-toxic, environmentally safe alternative to mercury for thermometers, with a melting point of 29.76degC compared to mercury's -38.83degC, making it suitable for measuring moderate temperature ranges. Gallium's lower vapor pressure and higher thermal expansion coefficient improve measurement accuracy and safety in clinical and laboratory thermometers.
Table of Comparison
| Property | Gallium | Mercury |
|---|---|---|
| Melting Point | 29.76 degC | -38.83 degC |
| Boiling Point | 2204 degC | 356.73 degC |
| Toxicity | Non-toxic | Highly toxic |
| Density | 5.91 g/cm3 | 13.53 g/cm3 |
| Thermal Expansion | Low | High |
| Visibility in Scale | Silvery, less visible | Shiny, highly visible |
| Environmental Impact | Eco-friendly | Polluting |
| Use in Thermometers | Alternative to mercury | Traditional standard |
Introduction to Gallium and Mercury Thermometers
Gallium thermometers utilize the metal gallium, known for its non-toxic, environmentally friendly properties and a melting point near room temperature, making it ideal for precise temperature measurements. Mercury thermometers contain liquid mercury, valued for its high density and consistent thermal expansion, but its toxicity and environmental hazards have led to reduced usage. Both types provide accurate temperature readings, but gallium thermometers offer safer handling and disposal advantages in modern applications.
Chemical Properties of Gallium vs Mercury
Gallium exhibits a melting point of approximately 29.76degC, allowing it to transition from solid to liquid near room temperature, unlike mercury, which remains liquid with a melting point of -38.83degC. Gallium is chemically inert and non-toxic, forming a protective oxide layer that prevents oxidation, whereas mercury is highly toxic and presents significant health and environmental hazards. Both metals possess high density and electrical conductivity, but gallium's lower vapor pressure reduces risks associated with evaporation compared to mercury in thermometric applications.
Temperature Measurement Range
Gallium offers a wider temperature measurement range for thermometers, typically from -19degC to 2204degC, compared to mercury's narrower range of -39degC to 357degC. Gallium's higher boiling point and ability to remain liquid at sub-zero temperatures make it ideal for extreme temperature applications. Mercury's toxicity limits its use despite stable readings within its suitable temperature range.
Environmental and Health Impacts
Gallium thermometers offer a safer and eco-friendly alternative to mercury thermometers due to gallium's low toxicity and non-volatile nature, reducing risks of mercury poisoning and environmental contamination. Mercury is a persistent pollutant that bioaccumulates in ecosystems, causing severe neurological and health issues in humans and wildlife upon exposure or disposal. Switching to gallium thermometers minimizes hazardous waste, promotes sustainability, and aligns with global efforts to phase out mercury-based devices under the Minamata Convention on Mercury.
Accuracy and Precision Comparison
Gallium thermometers provide superior accuracy and precision compared to mercury thermometers due to gallium's wider liquid range and lower thermal expansion variability. Gallium remains liquid at temperatures ranging from approximately 29.8degC to 2204degC, allowing for more consistent and reliable temperature readings, especially in fluctuating environments. Mercury's higher density and toxicity pose challenges for precise measurement calibration and safe handling, making gallium alloys a safer and more stable alternative in modern temperature sensing applications.
Durability and Longevity
Gallium thermometers exhibit superior durability compared to mercury due to their non-toxic, corrosion-resistant properties and resistance to freezing at low temperatures, enhancing longevity in varied environments. Mercury thermometers, while historically favored for their high density and stable thermal expansion, face challenges with toxicity and brittleness of glass encasements that may reduce lifespan. Gallium's extended operational temperature range and environmental safety contribute to longer-lasting, more reliable thermometer performance in modern applications.
Safety Considerations in Handling
Gallium is significantly safer than mercury for thermometers due to its non-toxic and environmentally friendly properties, reducing the risk of poisoning and contamination during handling or accidental breakage. Mercury, a heavy metal, poses serious health hazards including neurotoxicity and requires specialized cleanup procedures to prevent mercury vapor inhalation. Using gallium thermometers minimizes safety concerns, making them preferable in educational and household settings where accidental spills might occur.
Cost and Availability
Gallium thermometers are generally more expensive than mercury thermometers due to the higher cost and limited industrial-scale extraction of gallium. Mercury is abundant, widely available, and cheaper to produce, making traditional mercury thermometers more cost-effective for mass use. However, gallium offers a safer, non-toxic alternative despite its higher market price and less widespread availability.
Regulatory and Legal Restrictions
Gallium thermometers face fewer regulatory restrictions compared to mercury thermometers, which are heavily regulated due to mercury's toxicity and environmental hazards. Many countries enforce strict bans or limitations on mercury thermometer sales and disposal under international agreements like the Minamata Convention on Mercury. This legal landscape drives increasing adoption of gallium-based alternatives, which are deemed safer and compliant with environmental protection standards.
Future Trends in Thermometer Materials
Gallium-based thermometers present a promising future trend due to their non-toxicity, high thermal conductivity, and wide liquid range compared to mercury, which is restricted due to its toxicity and environmental hazards. Innovations in gallium alloys improve safety and accuracy in temperature measurement while addressing regulatory bans on mercury devices globally. Emerging research on nanostructured gallium composites aims to enhance responsiveness and durability, positioning gallium as a sustainable and efficient alternative in next-generation thermometer materials.
Infographic: Gallium vs Mercury for Thermometer
 azmater.com
azmater.com