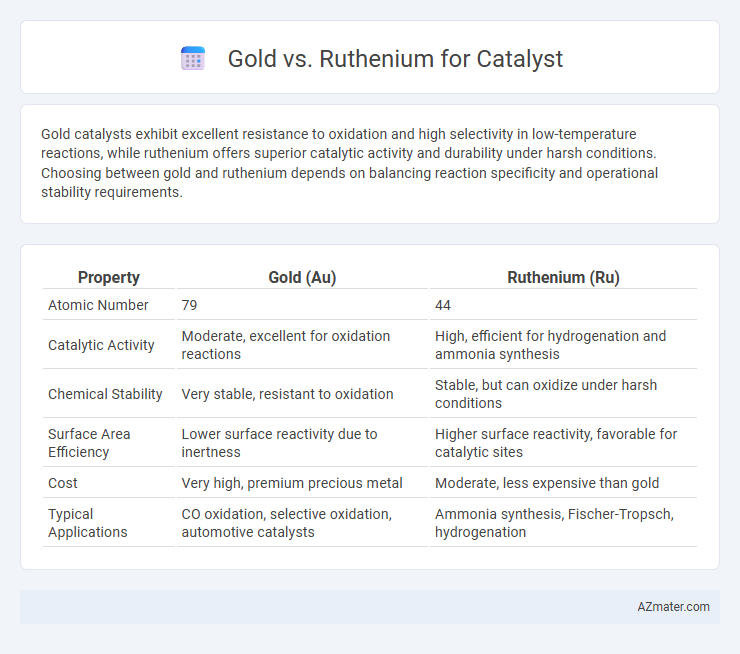
Gold catalysts exhibit excellent resistance to oxidation and high selectivity in low-temperature reactions, while ruthenium offers superior catalytic activity and durability under harsh conditions. Choosing between gold and ruthenium depends on balancing reaction specificity and operational stability requirements.
Table of Comparison
| Property | Gold (Au) | Ruthenium (Ru) |
|---|---|---|
| Atomic Number | 79 | 44 |
| Catalytic Activity | Moderate, excellent for oxidation reactions | High, efficient for hydrogenation and ammonia synthesis |
| Chemical Stability | Very stable, resistant to oxidation | Stable, but can oxidize under harsh conditions |
| Surface Area Efficiency | Lower surface reactivity due to inertness | Higher surface reactivity, favorable for catalytic sites |
| Cost | Very high, premium precious metal | Moderate, less expensive than gold |
| Typical Applications | CO oxidation, selective oxidation, automotive catalysts | Ammonia synthesis, Fischer-Tropsch, hydrogenation |
Introduction to Gold and Ruthenium as Catalysts
Gold and ruthenium are widely studied transition metals known for their distinctive catalytic properties in heterogeneous catalysis. Gold catalysts exhibit exceptional activity and selectivity in oxidation reactions and CO oxidation at low temperatures due to their unique electronic structure and stability. Ruthenium catalysts demonstrate remarkable efficiency in hydrogenation, ammonia synthesis, and water splitting reactions, benefiting from their ability to facilitate multiple electron transfer processes.
Chemical Properties Affecting Catalytic Performance
Gold exhibits high resistance to oxidation and remarkable stability under aerobic conditions, which preserves its catalytic activity in oxidation reactions. Ruthenium, possessing multiple accessible oxidation states, facilitates redox cycling and enhances catalytic efficiency in hydrogenation and ammonia synthesis. The intrinsic electronic structure of ruthenium allows stronger adsorption and activation of reactant molecules compared to gold, directly impacting turnover frequency and selectivity in catalytic processes.
Synthesis and Preparation Methods
Gold catalysts are typically synthesized through methods such as colloidal deposition, impregnation, and chemical vapor deposition, with precise control over nanoparticle size achieved by using stabilizing agents like citrate or polyvinyl alcohol. Ruthenium catalysts are commonly prepared via impregnation, co-precipitation, and sputtering techniques, often requiring reduction under hydrogen to activate the catalyst surface. Both metals benefit from support materials like silica or alumina to enhance dispersion and catalytic activity, but ruthenium's higher affinity for hydrogen often necessitates more rigorous preparation conditions to prevent sintering.
Catalytic Efficiency and Reaction Selectivity
Gold catalysts exhibit exceptional catalytic efficiency in oxidation reactions, particularly at low temperatures, due to their unique electronic properties and resistance to poisoning. Ruthenium catalysts demonstrate superior reaction selectivity in hydrogenation and ammonia synthesis processes, enabled by their ability to facilitate multiple reaction pathways with controlled intermediate binding. Comparative studies highlight ruthenium's higher turnover frequencies in complex organic transformations, while gold's inertness contributes to minimal by-product formation, making each metal optimal for specific catalytic applications.
Stability and Longevity Under Reaction Conditions
Ruthenium catalysts exhibit superior stability and longevity under harsh reaction conditions compared to gold, maintaining catalytic activity over extended cycles without significant degradation. Gold catalysts often suffer from sintering and surface oxidation, leading to diminished performance in high-temperature or oxidative environments. The robust electronic structure and resistance to poisoning make ruthenium an ideal choice for sustained catalytic reactions requiring long-term durability.
Applications in Industrial Catalysis
Gold catalysts exhibit exceptional performance in selective oxidation and hydrogenation reactions, prominently applied in fine chemical synthesis and environmental catalysis such as CO oxidation and VOC abatement. Ruthenium catalysts are widely utilized for ammonia synthesis, Fischer-Tropsch reactions, and hydrogenation of nitrogen-containing compounds due to their high activity and robustness under harsh industrial conditions. Both metals serve crucial roles in heterogeneous catalysis, with gold favored for low-temperature and selective processes while ruthenium excels in high-pressure, high-temperature industrial applications.
Environmental Impact and Sustainability
Gold catalysts exhibit high stability and resistance to corrosion, leading to longer catalyst lifespans and reduced waste generation. Ruthenium, while more abundant and less expensive, can pose toxicity risks and requires careful management to minimize environmental contamination. Sustainable catalytic processes favor gold due to its recyclability and minimal leaching, but ruthenium's lower resource intensity offers an alternative with proper environmental controls.
Cost and Availability Considerations
Gold catalysts offer high chemical stability and superior resistance to oxidation but come with significantly higher costs due to gold's rarity and market price volatility. Ruthenium, while less expensive and more abundant than gold, provides excellent catalytic activity particularly in hydrogenation reactions, making it a cost-effective alternative for industrial applications. Availability of ruthenium is linked to platinum group metal mining with more stable supply chains compared to gold, which faces frequent speculative price fluctuations impacting overall catalyst cost-efficiency.
Recent Advances in Gold and Ruthenium Catalysts
Recent advances in gold catalysts highlight their exceptional selectivity and stability in oxidation reactions, particularly for CO oxidation and alcohol transformations under mild conditions. Ruthenium catalysts demonstrate significant progress in hydrogenation and ammonia synthesis, benefiting from enhanced support interactions and nano-engineering that improve activity and resistance to deactivation. Comparative studies emphasize gold's superior performance in aerobic oxidations, while ruthenium excels in hydrogenation processes, reflecting their complementary roles in catalysis design.
Future Prospects and Emerging Research Directions
Gold catalysts exhibit exceptional selectivity and stability for oxidation reactions, while ruthenium catalysts demonstrate superior activity in hydrogenation and ammonia synthesis, positioning both as critical materials for sustainable chemical processes. Emerging research focuses on enhancing ruthenium's cost-effectiveness and durability through alloying and nanostructuring, whereas gold catalysts are being engineered at the atomic scale to improve efficiency in carbon dioxide reduction and environmental remediation. Future prospects include integrating gold and ruthenium into hybrid catalytic systems to leverage their complementary properties, advancing renewable energy conversion, and green chemical production technologies.
Infographic: Gold vs Ruthenium for Catalyst
 azmater.com
azmater.com