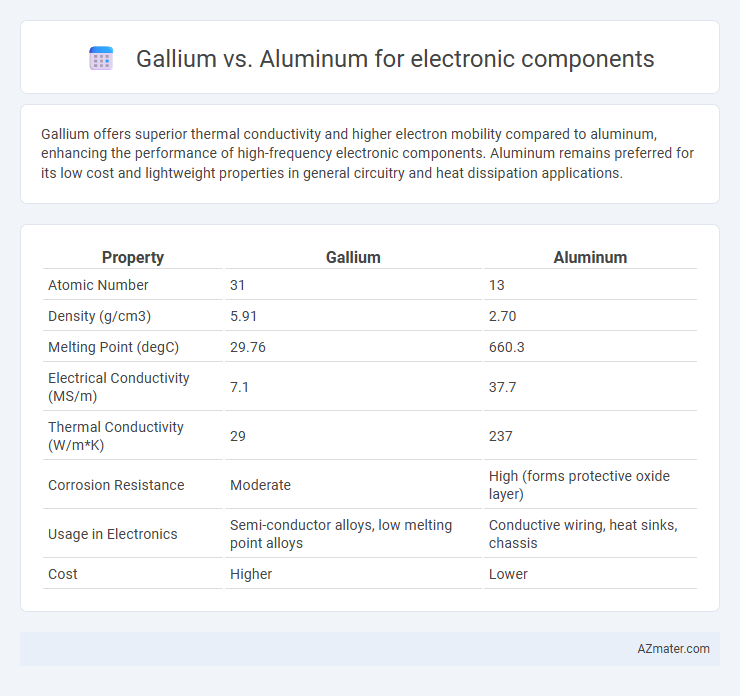
Gallium offers superior thermal conductivity and higher electron mobility compared to aluminum, enhancing the performance of high-frequency electronic components. Aluminum remains preferred for its low cost and lightweight properties in general circuitry and heat dissipation applications.
Table of Comparison
| Property | Gallium | Aluminum |
|---|---|---|
| Atomic Number | 31 | 13 |
| Density (g/cm3) | 5.91 | 2.70 |
| Melting Point (degC) | 29.76 | 660.3 |
| Electrical Conductivity (MS/m) | 7.1 | 37.7 |
| Thermal Conductivity (W/m*K) | 29 | 237 |
| Corrosion Resistance | Moderate | High (forms protective oxide layer) |
| Usage in Electronics | Semi-conductor alloys, low melting point alloys | Conductive wiring, heat sinks, chassis |
| Cost | Higher | Lower |
Introduction to Gallium and Aluminum in Electronics
Gallium and aluminum both play crucial roles in electronic components, with gallium primarily used in semiconductors like gallium arsenide (GaAs) for high-speed and optoelectronic applications, while aluminum is widely utilized for electrical wiring and conductive interconnects due to its excellent conductivity and lightweight properties. Gallium's unique characteristics enable efficient performance in microwave frequency devices and LEDs, whereas aluminum's abundance and cost-effectiveness make it a standard choice in circuit boards and heat dissipation elements. Understanding the distinct electrical, thermal, and mechanical properties of gallium and aluminum is essential for optimizing design and function in advanced electronic systems.
Physical and Chemical Properties Comparison
Gallium exhibits a melting point of 29.76degC, significantly lower than aluminum's 660.3degC, making it useful for low-temperature soldering applications in electronic components. Chemically, gallium is less reactive than aluminum, resisting oxidation longer but forming a protective oxide layer that enhances corrosion resistance. Aluminum offers superior electrical conductivity and mechanical strength, while gallium's unique thermal expansion and softness enable specialized roles in semiconductor fabrication and thermal interface materials.
Electrical Conductivity Differences
Gallium exhibits significantly lower electrical conductivity compared to aluminum, with gallium's conductivity around 7.8 million Siemens per meter (S/m) versus aluminum's approximately 37.7 million S/m. This difference makes aluminum a preferred choice for electronic components requiring efficient electrical conduction, such as wiring and connectors. However, gallium's unique semiconductor properties are leveraged in specialized applications like gallium arsenide (GaAs) devices, where electrical conductivity is tuned through compound formation rather than pure metallic behavior.
Thermal Performance: Gallium vs Aluminum
Gallium boasts superior thermal conductivity compared to aluminum, enabling more efficient heat dissipation in electronic components and reducing the risk of overheating. Its melting point around 29.76degC allows for unique thermal interface applications, enhancing thermal management in sensitive devices. Aluminum, while lighter and more abundant, has lower thermal conductivity, typically around 205 W/m*K, making gallium a preferred choice in high-performance heat transfer scenarios.
Corrosion Resistance and Longevity
Gallium exhibits superior corrosion resistance compared to aluminum, particularly in electronic components exposed to harsh environments or moisture, due to its stable oxide layer that prevents rapid degradation. Aluminum, while lightweight and widely used, is more susceptible to corrosion, especially in chloride-rich settings, which can compromise the longevity of electronic parts. Gallium's enhanced resistance contributes to longer-lasting, more reliable electronics, making it preferable for applications demanding durability and minimal maintenance.
Cost Analysis and Availability
Gallium, primarily used in high-performance semiconductors, is significantly more expensive and less abundant than aluminum, which is widely available and cost-effective for general electronic components. The rarity of gallium, extracted mainly as a byproduct of mining bauxite and zinc ores, drives up its cost compared to aluminum's abundant global reserves and mature extraction technologies. Manufacturers prioritize aluminum for bulk electronic applications due to its affordability and availability, reserving gallium for specialized uses like gallium arsenide (GaAs) components where superior electronic properties justify the higher expense.
Application Suitability in Electronic Components
Gallium-based materials, notably gallium arsenide (GaAs), excel in high-frequency and high-speed electronic components due to superior electron mobility compared to aluminum-based components, which are primarily used in standard semiconductor applications. Gallium alloys are preferred in optoelectronics, such as LEDs and laser diodes, since they provide better performance in light emission and detection. Aluminum's lower cost and ease of integration make it suitable for conductive pathways and heat sinks but less optimal for high-frequency applications where gallium compounds dominate.
Environmental Impact and Sustainability
Gallium offers a lower environmental footprint than aluminum in electronic components due to its reduced energy consumption during extraction and processing, contributing to less greenhouse gas emissions. Aluminum is abundant but requires high-energy electrolytic refining, leading to significant environmental degradation and carbon output. Using gallium supports sustainable electronics by minimizing resource depletion and enabling more efficient recycling compared to aluminum-based components.
Technological Advancements and Innovations
Gallium's use in electronic components has driven significant technological advancements due to its superior electron mobility and direct bandgap properties, enabling high-speed and high-frequency devices like gallium arsenide (GaAs) semiconductors. Compared to aluminum, which is primarily used for wiring and packaging, gallium-based materials facilitate innovations in optoelectronics, such as LEDs and laser diodes, enhancing device efficiency and performance. Continued research in gallium compounds supports next-generation applications in 5G technology and high-power electronics, outperforming aluminum's capabilities in semiconductor innovation.
Future Trends in Electronic Material Selection
Gallium-based compounds, such as gallium nitride (GaN), are rapidly replacing aluminum in high-performance electronic components due to their superior thermal conductivity, higher electron mobility, and efficiency in power electronics and RF applications. The future trend in electronic material selection emphasizes GaN for next-generation devices in 5G, electric vehicles, and renewable energy systems because of its ability to operate at higher voltages and frequencies with lower energy loss. Aluminum remains essential for cost-effective applications, but the demand for gallium-enhanced materials is growing steadily as industries prioritize performance and sustainability.
Infographic: Gallium vs Aluminum for Electronic component
 azmater.com
azmater.com