Iridium offers superior corrosion resistance and high-temperature stability compared to palladium, making it ideal for harsh chemical process environments. Palladium excels in catalytic hydrogenation reactions due to its exceptional hydrogen absorption properties and cost-effectiveness.
Table of Comparison
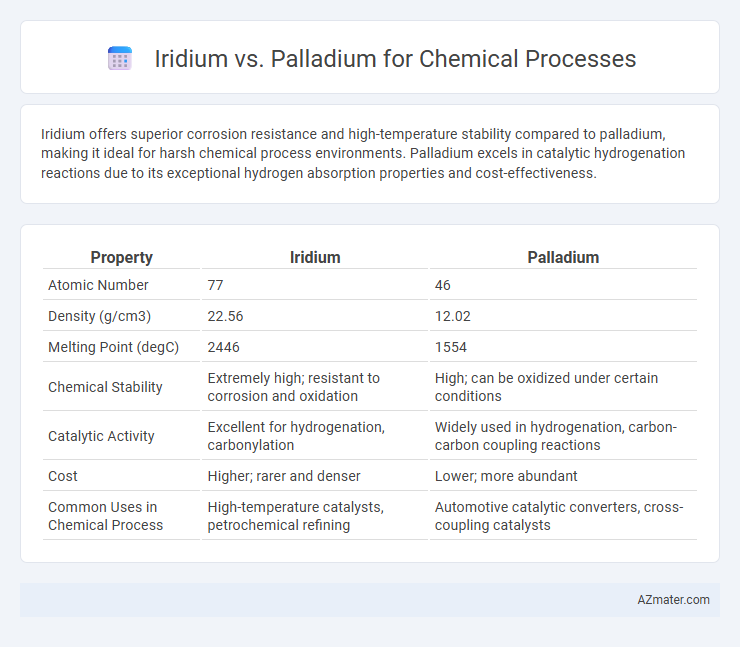
| Property | Iridium | Palladium |
|---|---|---|
| Atomic Number | 77 | 46 |
| Density (g/cm3) | 22.56 | 12.02 |
| Melting Point (degC) | 2446 | 1554 |
| Chemical Stability | Extremely high; resistant to corrosion and oxidation | High; can be oxidized under certain conditions |
| Catalytic Activity | Excellent for hydrogenation, carbonylation | Widely used in hydrogenation, carbon-carbon coupling reactions |
| Cost | Higher; rarer and denser | Lower; more abundant |
| Common Uses in Chemical Process | High-temperature catalysts, petrochemical refining | Automotive catalytic converters, cross-coupling catalysts |
Introduction to Iridium and Palladium in Chemical Processes
Iridium and palladium are crucial catalysts in chemical processes, valued for their unique properties and catalytic efficiencies. Iridium is renowned for its durability and effectiveness in high-temperature reactions and oxidation catalysts, particularly in water-splitting and hydrogenation reactions. Palladium excels in catalytic hydrogenation and carbon-carbon coupling reactions, widely used in pharmaceutical synthesis and organic chemistry due to its high selectivity and reusability.
Atomic and Chemical Properties Comparison
Iridium and palladium exhibit distinct atomic and chemical properties influencing their roles in chemical processes; iridium has an atomic number of 77 and a melting point of 2446degC, showcasing superior thermal stability compared to palladium's atomic number 46 and melting point of 1554degC. Chemically, iridium forms more resistant and stable complexes, often utilized in high-temperature catalytic reactions, while palladium is renowned for its exceptional catalytic activity in hydrogenation and carbon-carbon coupling reactions due to its ability to easily adsorb hydrogen. The electron configuration of iridium [Xe]4f^14 5d^7 6s^2 contributes to its inertness and durability in harsh conditions, whereas palladium's [Kr]4d^10 configuration allows for flexible oxidation states, enhancing its versatility in diverse catalytic applications.
Catalytic Efficiency: Iridium vs Palladium
Iridium demonstrates superior catalytic efficiency in chemical processes involving hydrogenation and C-H activation due to its high stability and resistance to poisoning. Palladium is highly effective for carbon-carbon coupling reactions such as Suzuki and Heck, offering excellent selectivity and faster reaction rates in cross-coupling catalysis. While iridium excels in harsh reaction conditions and diverse substrate compatibility, palladium remains the preferred choice for large-scale industrial applications because of its cost-effectiveness and established catalytic performance.
Industrial Applications in Chemical Processing
Iridium exhibits exceptional corrosion resistance and thermal stability, making it ideal for harsh chemical process environments such as acid catalysis and high-temperature reactions. Palladium offers superior hydrogen absorption properties and catalytic efficiency in hydrogenation and dehydrogenation processes, widely utilized in refining and petrochemical industries. Industrial applications favor iridium for durability in aggressive media, while palladium is preferred for its catalytic versatility in organic synthesis and fuel cell technologies.
Cost and Market Availability Analysis
Iridium offers exceptional corrosion resistance and catalytic stability in harsh chemical processes but typically commands a higher market price due to its rarity and lower annual production of around 7 tons compared to palladium's 200 tons. Palladium, with its broader industrial applications and larger market supply, is more cost-effective and readily available, making it a preferred choice for large-scale chemical reactions despite slightly lower durability in aggressive environments. Market volatility for both metals is influenced by factors such as mining output, geopolitical stability, and demand from automotive and electronics sectors, directly impacting the cost-efficiency of chemical process catalysis.
Longevity and Stability Under Reaction Conditions
Iridium exhibits superior longevity and stability under harsh chemical process conditions due to its exceptional resistance to oxidation and corrosion, making it ideal for high-temperature and strongly acidic or oxidative environments. Palladium, while highly active catalytically, tends to degrade faster under prolonged exposure to reactive media, particularly in oxidizing atmospheres, leading to catalyst deactivation and reduced lifespan. Therefore, iridium's robustness significantly enhances catalyst durability and process efficiency in demanding chemical reactions.
Environmental Impact and Sustainability
Iridium and palladium play critical roles in chemical processes, especially in catalysis, with significant differences in environmental impact and sustainability. Iridium's high durability and resistance to corrosion reduce catalyst degradation, minimizing waste and the need for frequent replacement, which enhances long-term sustainability. Palladium, although more abundant and widely used, often requires extensive mining practices that lead to greater environmental disturbance and higher energy consumption, making iridium a more environmentally favorable choice despite its scarcity and cost.
Selectivity and Reaction Pathways
Iridium catalysts exhibit superior selectivity in hydrogenation reactions due to their ability to favor specific reaction pathways, minimizing byproduct formation and enhancing yield purity. Palladium, while effective in various catalytic processes, tends to activate multiple pathways, often resulting in lower selectivity and more complex product mixtures. The choice of iridium over palladium is critical in chemical processes where precise control over reaction pathways directly impacts overall efficiency and product consistency.
Scalability for Industrial Use
Iridium demonstrates superior scalability for industrial chemical processes due to its exceptional corrosion resistance and stable catalytic activity under extreme conditions, making it ideal for high-volume production. Palladium offers cost advantages and excellent catalytic performance in hydrogenation and carbon-carbon coupling reactions but faces challenges in durability and catalyst poisoning in large-scale applications. The choice between iridium and palladium hinges on balancing scalability demands with economic and operational factors in industrial chemical manufacturing.
Future Trends in Chemical Process Catalysis
Iridium and palladium remain crucial catalysts in chemical process catalysis, with iridium excelling in hydrogenation and oxidation reactions due to its high stability and resistance to sintering, while palladium is favored for carbon-carbon coupling and selective hydrogenation processes. Future trends indicate a shift towards developing bimetallic and alloy catalysts combining iridium and palladium to enhance catalytic efficiency, selectivity, and durability under harsh reaction conditions. Advances in nanoscale catalyst design and computational modeling are driving the optimization of these metals' surface properties, enabling sustainable and energy-efficient chemical processes in the pharmaceutical, petrochemical, and fine chemical industries.
Infographic: Iridium vs Palladium for Chemical Process
 azmater.com
azmater.com