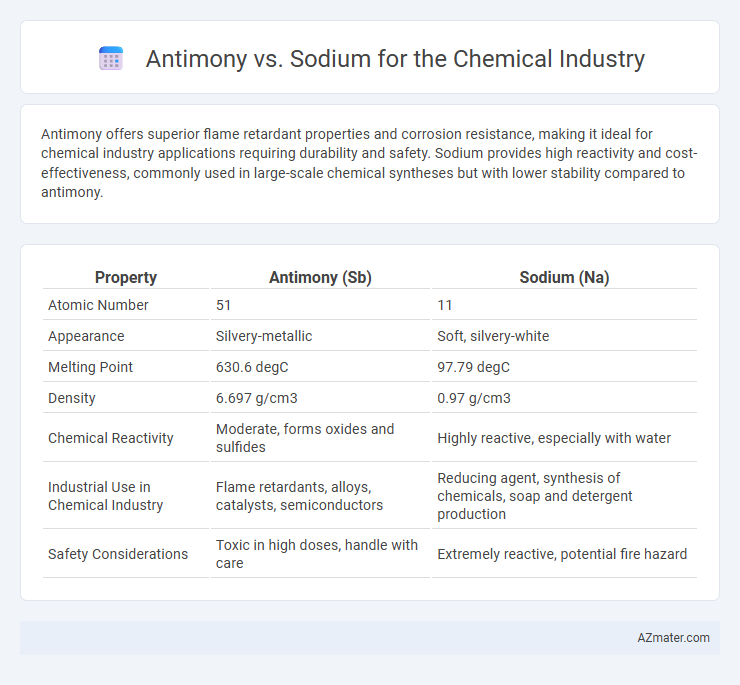
Antimony offers superior flame retardant properties and corrosion resistance, making it ideal for chemical industry applications requiring durability and safety. Sodium provides high reactivity and cost-effectiveness, commonly used in large-scale chemical syntheses but with lower stability compared to antimony.
Table of Comparison
| Property | Antimony (Sb) | Sodium (Na) |
|---|---|---|
| Atomic Number | 51 | 11 |
| Appearance | Silvery-metallic | Soft, silvery-white |
| Melting Point | 630.6 degC | 97.79 degC |
| Density | 6.697 g/cm3 | 0.97 g/cm3 |
| Chemical Reactivity | Moderate, forms oxides and sulfides | Highly reactive, especially with water |
| Industrial Use in Chemical Industry | Flame retardants, alloys, catalysts, semiconductors | Reducing agent, synthesis of chemicals, soap and detergent production |
| Safety Considerations | Toxic in high doses, handle with care | Extremely reactive, potential fire hazard |
Introduction to Antimony and Sodium
Antimony (Sb), a metalloid with atomic number 51, plays a crucial role in flame retardants, lead alloys, and semiconductors within the chemical industry. Sodium (Na), an alkali metal with atomic number 11, is essential for producing synthetic detergents, paper pulp, and sodium compounds like sodium hydroxide and sodium carbonate. Both elements possess unique properties that drive their distinct industrial applications, emphasizing their importance in manufacturing and chemical processes.
Chemical Properties Comparison
Antimony exhibits a stable oxidation state of +3 or +5, showcasing metalloid characteristics with moderate electrical conductivity and resistance to oxidation, whereas sodium is a highly reactive alkali metal with a single +1 oxidation state and strong reducing properties. Antimony's semimetal nature enables its use in flame retardants and alloys, while sodium's high reactivity and low electronegativity make it crucial for synthesis of sodium compounds and as a reducing agent in chemical reactions. The significant differences in electronegativity, atomic radius, and electron configuration between antimony (Sb) and sodium (Na) dictate their distinct chemical behaviors and industrial applications.
Industrial Applications Overview
Antimony is predominantly used in flame retardants, lead-acid battery alloys, and as a catalyst in the production of polyethylene terephthalate (PET). Sodium's industrial applications include large-scale chemical syntheses such as the production of synthetic rubber, pharmaceuticals, and as a strong reducing agent in organic reactions. Both elements are critical in the chemical industry, with antimony enhancing material properties and sodium enabling key chemical transformations.
Antimony in Chemical Processes
Antimony plays a critical role in the chemical industry as a flame retardant and catalyst, particularly in the production of polyethylene terephthalate (PET) plastics and synthetic fibers. Its unique properties enable enhanced thermal stability and improved material performance compared to sodium, which is primarily utilized as a strong reducing agent and in the manufacture of chemicals like sodium hydroxide. The catalytic efficiency and flame retardant capabilities of antimony compounds make them indispensable for advanced chemical processes and industrial applications.
Sodium in Chemical Manufacturing
Sodium plays a crucial role in chemical manufacturing due to its high reactivity and ability to form various compounds such as sodium hydroxide, sodium carbonate, and sodium chloride, which are essential in producing glass, paper, and detergents. Unlike antimony, which is primarily used as a flame retardant or alloying element, sodium's versatility as a reducing agent and its widespread application in organic synthesis make it indispensable in the chemical industry. The abundance and cost-effectiveness of sodium further enhance its preference over antimony for large-scale chemical processes.
Reactivity and Safety Considerations
Antimony exhibits low reactivity with water and air, making it stable and safe for use in high-temperature chemical processes, whereas sodium is highly reactive, especially with water, releasing hydrogen gas and heat that pose significant explosion risks. Sodium's vigorous reactivity necessitates stringent safety protocols and inert atmospheres to prevent hazardous reactions in industrial applications. The chemical industry often selects antimony for processes requiring stability and reduced safety hazards, while sodium is chosen for its strong reducing properties despite its handling challenges.
Environmental Impact of Antimony vs Sodium
Antimony's environmental impact is primarily linked to its mining and refining processes that release toxic byproducts like antimony trioxide, which can contaminate soil and water, posing risks to ecosystems and human health. Sodium, being an abundant alkali metal, generally presents lower environmental risks during extraction, although its reactive nature requires careful handling to prevent hazardous releases and chemical accidents. The chemical industry's preference often hinges on balancing antimony's toxicological concerns with sodium's combustion-related hazards, emphasizing sustainable sourcing and waste management practices to mitigate overall environmental impact.
Economic Factors and Market Trends
Antimony commands higher market value due to its critical role in flame retardants, batteries, and alloys, with global production heavily concentrated in China, impacting supply stability and price volatility. Sodium, widely produced through abundant natural resources and electrolysis methods, maintains lower costs and steady demand primarily in chemical manufacturing, glass production, and pharmaceuticals. Economic trends show growing interest in antimony recycling and strategic stockpiling amid geopolitical tensions, while sodium market growth aligns with expanding industrial applications and sustainable chemistry initiatives.
Challenges in Handling and Storage
Antimony requires careful handling due to its toxic properties and propensity to oxidize when exposed to air, necessitating storage in tightly sealed containers under inert atmospheres. Sodium is highly reactive, especially with water and moisture, demanding rigorous storage in oil or sealed environments to prevent violent reactions and fire hazards. Both elements pose significant safety challenges in industrial settings, requiring specialized protocols for containment, ventilation, and personnel protection to mitigate risks.
Future Prospects in the Chemical Industry
Antimony and sodium each hold unique future prospects in the chemical industry due to their distinct properties and applications. Antimony's role in flame retardants, lead-acid batteries, and semiconductors positions it as a critical material amid rising demand for safety and energy storage technologies. Sodium's increasing use in sustainable energy solutions, such as sodium-ion batteries and green chemical processes, highlights its potential to drive innovation in low-cost, environmentally friendly industrial applications.
Infographic: Antimony vs Sodium for Chemical Industry
 azmater.com
azmater.com