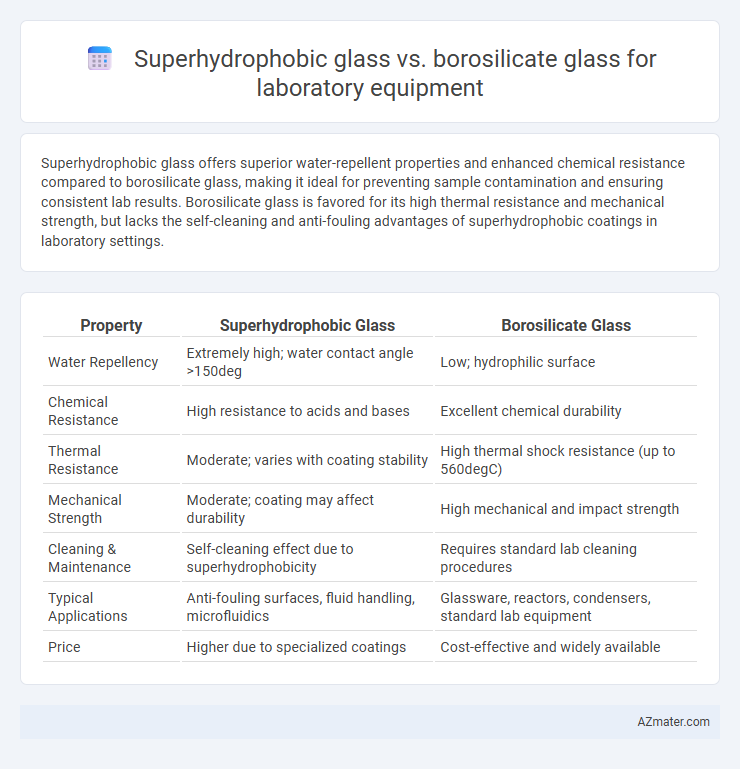
Superhydrophobic glass offers superior water-repellent properties and enhanced chemical resistance compared to borosilicate glass, making it ideal for preventing sample contamination and ensuring consistent lab results. Borosilicate glass is favored for its high thermal resistance and mechanical strength, but lacks the self-cleaning and anti-fouling advantages of superhydrophobic coatings in laboratory settings.
Table of Comparison
| Property | Superhydrophobic Glass | Borosilicate Glass |
|---|---|---|
| Water Repellency | Extremely high; water contact angle >150deg | Low; hydrophilic surface |
| Chemical Resistance | High resistance to acids and bases | Excellent chemical durability |
| Thermal Resistance | Moderate; varies with coating stability | High thermal shock resistance (up to 560degC) |
| Mechanical Strength | Moderate; coating may affect durability | High mechanical and impact strength |
| Cleaning & Maintenance | Self-cleaning effect due to superhydrophobicity | Requires standard lab cleaning procedures |
| Typical Applications | Anti-fouling surfaces, fluid handling, microfluidics | Glassware, reactors, condensers, standard lab equipment |
| Price | Higher due to specialized coatings | Cost-effective and widely available |
Introduction to Laboratory Glass Types
Superhydrophobic glass provides enhanced water repellency and self-cleaning properties, making it ideal for preventing contamination and residue build-up in laboratory environments. Borosilicate glass is widely favored for its exceptional thermal resistance and chemical durability, ensuring reliable performance under extreme temperature fluctuations and corrosive reagents. Selecting between superhydrophobic and borosilicate glass depends on the specific laboratory application requirements, such as hydrophobicity versus temperature and chemical resistance.
What is Superhydrophobic Glass?
Superhydrophobic glass is a specially treated surface that exhibits extreme water repellency, characterized by water contact angles greater than 150 degrees, preventing liquid adherence and contamination. In laboratory settings, this glass offers superior resistance to chemical spills and easier cleaning compared to traditional materials. Borosilicate glass, known for its thermal and chemical durability, lacks this water-repellent property, making superhydrophobic glass advantageous for experiments requiring minimal liquid residue and enhanced surface protection.
Overview of Borosilicate Glass Properties
Borosilicate glass is distinguished by its exceptional thermal resistance, low thermal expansion coefficient (approximately 3.3 x 10^-6 /degC), and high chemical durability, making it ideal for laboratory equipment exposed to rapid temperature changes and corrosive substances. Its composition typically includes silica and boron oxide, which contribute to its robustness against thermal shock and chemical attack. These properties ensure borosilicate glass maintains structural integrity and clarity under harsh experimental conditions, outperforming standard glass types in laboratory applications.
Chemical Resistance Comparison
Superhydrophobic glass exhibits superior chemical resistance due to its specialized surface coating that repels a wide range of corrosive substances, minimizing surface degradation and contamination risks in laboratory settings. Borosilicate glass, while highly resistant to thermal shock and many chemicals, can be more susceptible to acid attack and alkali damage over prolonged exposure compared to superhydrophobic coatings. The enhanced chemical durability of superhydrophobic glass makes it preferable for handling aggressive solvents and reagents, ensuring longer-lasting lab equipment performance.
Thermal Stability and Temperature Performance
Superhydrophobic glass typically exhibits enhanced water repellency but generally lacks the high thermal stability of borosilicate glass, which can withstand temperatures up to 500degC without deformation. Borosilicate glass remains the preferred choice for laboratory equipment requiring consistent temperature performance and resistance to thermal shock due to its low coefficient of thermal expansion. While superhydrophobic coatings can be applied to borosilicate glass, the base material's inherent thermal stability ensures reliable performance in high-temperature laboratory applications.
Surface Wettability and Cleaning Ease
Superhydrophobic glass exhibits extreme water repellency with contact angles above 150deg, significantly reducing surface wettability compared to borosilicate glass, which has moderate wettability with contact angles typically around 30-40deg. This low surface energy in superhydrophobic glass minimizes liquid adhesion, allowing contaminants and residues to be easily removed by simple rinsing or airflow, enhancing cleaning efficiency. Borosilicate glass, while chemically resistant and thermally stable, tends to retain residues due to higher surface wettability, requiring more rigorous cleaning protocols to maintain purity in laboratory applications.
Durability and Mechanical Strength
Superhydrophobic glass offers enhanced durability against chemical corrosion and water damage, outperforming traditional borosilicate glass in environments requiring frequent liquid exposure. Borosilicate glass provides superior mechanical strength and thermal resistance, making it ideal for high-temperature laboratory applications. The choice depends on the specific lab usage, with superhydrophobic glass excelling in anti-contamination and ease of cleaning while borosilicate glass withstands thermal shock and mechanical stress effectively.
Application Suitability in Laboratory Settings
Superhydrophobic glass offers exceptional water repellency and chemical resistance, making it ideal for applications requiring minimal solvent retention and easy cleaning in laboratory settings. Borosilicate glass, known for its high thermal resistance and chemical durability, excels in high-temperature experiments and corrosive chemical handling. For applications demanding rapid fluid drainage and reduced contamination risk, superhydrophobic glass is advantageous, whereas borosilicate glass remains preferred for general-purpose labware exposed to heat and harsh reagents.
Cost Analysis and Availability
Superhydrophobic glass, known for its self-cleaning and anti-fouling properties, typically incurs higher costs due to advanced surface treatments and limited manufacturing scale compared to widely produced borosilicate glass. Borosilicate glass remains more cost-effective and readily available, favored in laboratories for its chemical resistance, thermal stability, and mass production. Cost analysis reveals borosilicate glass is a practical choice for routine laboratory equipment, while superhydrophobic glass's premium cost limits its use to specialized applications requiring enhanced surface properties.
Final Recommendations for Laboratory Use
Superhydrophobic glass offers superior water-repellent properties, reducing contamination and facilitating easier cleaning, making it ideal for applications requiring minimal sample adhesion. Borosilicate glass excels in thermal resistance, chemical durability, and mechanical strength, suitable for high-temperature reactions and aggressive chemical environments. For laboratory use, choose superhydrophobic glass when non-stick surfaces and contamination control are priorities, while borosilicate glass remains the preferred option for general-purpose lab glassware due to its robustness and versatility.
Infographic: Superhydrophobic glass vs Borosilicate glass for Laboratory equipment
 azmater.com
azmater.com