Borosilicate glass offers superior thermal resistance and chemical durability compared to water glass, making it ideal for laboratory beakers exposed to high temperatures and corrosive substances. Water glass, composed primarily of sodium silicate, lacks the heat tolerance and chemical stability needed for precise lab applications.
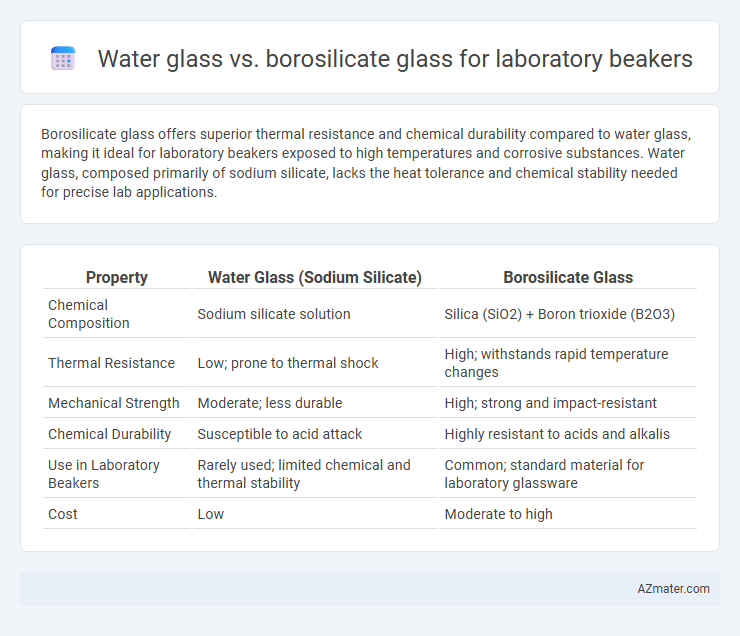
Table of Comparison
| Property | Water Glass (Sodium Silicate) | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Sodium silicate solution | Silica (SiO2) + Boron trioxide (B2O3) |
| Thermal Resistance | Low; prone to thermal shock | High; withstands rapid temperature changes |
| Mechanical Strength | Moderate; less durable | High; strong and impact-resistant |
| Chemical Durability | Susceptible to acid attack | Highly resistant to acids and alkalis |
| Use in Laboratory Beakers | Rarely used; limited chemical and thermal stability | Common; standard material for laboratory glassware |
| Cost | Low | Moderate to high |
Introduction: Importance of Choosing the Right Laboratory Beaker
Selecting the appropriate laboratory beaker significantly impacts experimental accuracy and safety, with water glass and borosilicate glass being two primary options. Borosilicate glass offers superior thermal resistance and chemical durability, making it ideal for high-temperature reactions and exposure to corrosive substances. Water glass, while more affordable, lacks the thermal stability and chemical resistance required for rigorous laboratory applications.
What is Water Glass? Composition and Properties
Water glass, also known as sodium silicate, is a compound composed primarily of silica (SiO2) and sodium oxide (Na2O) in varying ratios. It exhibits high thermal stability, excellent adhesive properties, and resistance to water and alkalis, making it useful in various industrial and laboratory applications. Unlike borosilicate glass, water glass is typically used as a coating or adhesive rather than a standalone material for laboratory beakers due to its distinct chemical and physical characteristics.
What is Borosilicate Glass? Composition and Advantages
Borosilicate glass, widely used in laboratory beakers, is composed primarily of silica (about 80%) and boron trioxide (around 13%), which gives it superior thermal resistance and chemical durability compared to regular water glass made mostly of soda-lime glass. Its low coefficient of thermal expansion (approximately 3.3 x 10^-6 /degC) minimizes the risk of thermal shock, making it ideal for high-temperature experiments and rapid temperature changes. The chemical inertness of borosilicate glass ensures minimal interaction with reactive substances, enhancing safety and reliability in precise laboratory applications.
Thermal Resistance: Water Glass vs Borosilicate Glass
Borosilicate glass offers superior thermal resistance compared to water glass, with a low coefficient of thermal expansion around 3.3 x 10^-6 /degC, allowing it to withstand rapid temperature changes without cracking. Water glass, primarily sodium silicate, has lower thermal stability and is prone to deformation or failure under high heat or thermal shock. Laboratory beakers made from borosilicate glass are preferred for high-temperature experiments due to their durability and resistance to thermal stress.
Chemical Durability and Reactivity Comparison
Water glass, primarily composed of sodium silicate, exhibits moderate chemical durability but tends to be more reactive with acidic substances, making it less suitable for aggressive chemical environments. Borosilicate glass, characterized by its low coefficient of thermal expansion and high resistance to chemical corrosion, offers superior chemical durability and minimal reactivity, ensuring reliable performance in laboratory beakers exposed to a wide range of chemicals. This enhanced chemical resilience of borosilicate glass reduces contamination risks and extends the lifespan of laboratory equipment compared to water glass.
Mechanical Strength: Impact and Scratch Resistance
Borosilicate glass exhibits superior mechanical strength compared to water glass, with higher resistance to impact and scratches, making it ideal for laboratory beakers subjected to frequent handling and thermal stress. Its low coefficient of thermal expansion reduces the likelihood of cracking under sudden temperature changes, whereas water glass is more prone to surface damage and fractures. Lab equipment made from borosilicate glass maintains durability and clarity over time, providing reliable performance in rigorous experimental conditions.
Cost and Availability in Laboratories
Water glass laboratory beakers are generally more cost-effective and widely available due to their simpler manufacturing process and common usage in basic experiments. Borosilicate glass beakers, though more expensive, offer superior thermal and chemical resistance, making them essential for high-precision or high-temperature applications. Laboratories with budget constraints often opt for water glass, while research facilities prioritize borosilicate glass for its durability and performance despite the higher cost.
Safety Considerations: Breakage and Hazards
Borosilicate glass laboratory beakers exhibit superior resistance to thermal shock and mechanical stress compared to regular water glass, reducing the risk of breakage during experiments. Water glass, often made from soda-lime glass, is more prone to cracking under rapid temperature changes, increasing hazards such as chemical spills and injury from shattered glass. Prioritizing borosilicate glass enhances overall lab safety by minimizing breakage incidents and exposure to potentially harmful substances.
Typical Applications for Each Glass Type
Water glass laboratory beakers, made from soda-lime glass, are typically used for less demanding applications such as mixing, simple chemical reactions, and short-term storage due to their lower thermal resistance and chemical durability. Borosilicate glass beakers are preferred in high-precision laboratory settings involving strong acids, bases, and high-temperature experiments because of their superior thermal shock resistance and chemical inertness. Research labs, pharmaceutical industries, and industrial chemical processing rely heavily on borosilicate glass for experiments requiring durability and accuracy under rigorous conditions.
Conclusion: Selecting the Best Glass for Your Laboratory Needs
Borosilicate glass is preferred for laboratory beakers due to its superior thermal resistance, chemical durability, and minimal thermal expansion, making it ideal for high-temperature experiments and reactive chemical exposure. Water glass, primarily sodium silicate, lacks the necessary thermal stability and chemical resistance for rigorous laboratory use, limiting its suitability to non-critical applications. Selecting borosilicate glass ensures reliability, safety, and longevity in versatile laboratory environments.
Infographic: Water glass vs Borosilicate glass for Laboratory beaker
 azmater.com
azmater.com