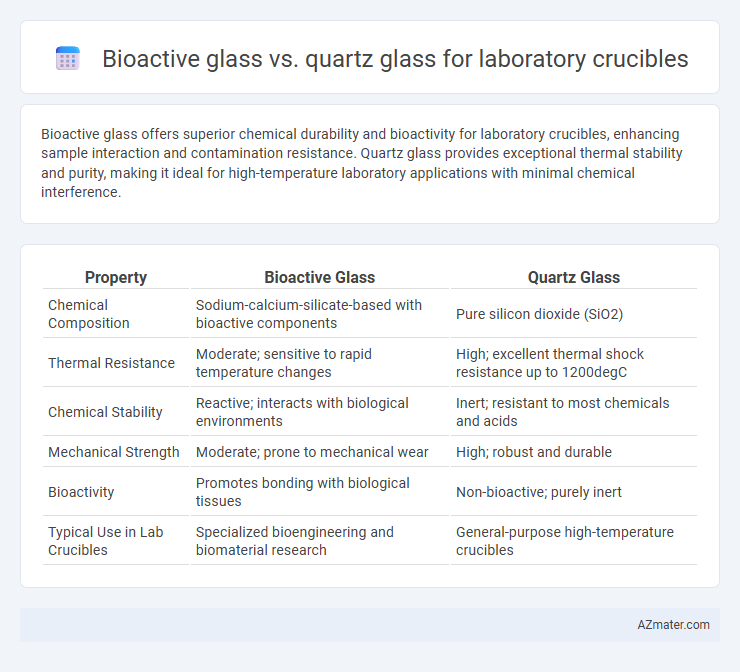
Bioactive glass offers superior chemical durability and bioactivity for laboratory crucibles, enhancing sample interaction and contamination resistance. Quartz glass provides exceptional thermal stability and purity, making it ideal for high-temperature laboratory applications with minimal chemical interference.
Table of Comparison
| Property | Bioactive Glass | Quartz Glass |
|---|---|---|
| Chemical Composition | Sodium-calcium-silicate-based with bioactive components | Pure silicon dioxide (SiO2) |
| Thermal Resistance | Moderate; sensitive to rapid temperature changes | High; excellent thermal shock resistance up to 1200degC |
| Chemical Stability | Reactive; interacts with biological environments | Inert; resistant to most chemicals and acids |
| Mechanical Strength | Moderate; prone to mechanical wear | High; robust and durable |
| Bioactivity | Promotes bonding with biological tissues | Non-bioactive; purely inert |
| Typical Use in Lab Crucibles | Specialized bioengineering and biomaterial research | General-purpose high-temperature crucibles |
Introduction to Laboratory Crucibles
Laboratory crucibles require materials with excellent thermal stability and chemical resistance, making bioactive glass and quartz glass common choices. Quartz glass offers superior thermal shock resistance and purity, essential for high-temperature reactions, while bioactive glass provides unique bio-compatibility and controlled dissolution properties. Selecting the appropriate crucible material depends on the specific experimental conditions and chemical interactions anticipated.
Overview of Bioactive Glass
Bioactive glass, known for its enhanced biocompatibility and reactive surface properties, is increasingly utilized in laboratory crucibles to promote bio-interactive experiments. Unlike quartz glass, which primarily offers high thermal stability and chemical inertness, bioactive glass facilitates biological integration and controlled ion release, making it ideal for biomedical research applications. Its unique composition reacts with physiological environments, enabling precise experimental outcomes in tissue engineering and drug delivery studies.
Overview of Quartz Glass
Quartz glass, also known as fused silica, offers exceptional thermal stability and chemical inertness, making it ideal for high-temperature laboratory crucibles. Its high melting point above 1700degC and resistance to most acids ensure durability during aggressive chemical reactions and thermal cycling. Unlike bioactive glass, quartz glass remains non-reactive, providing a pure, contamination-free environment essential for precise laboratory analyses.
Thermal Stability Comparison
Bioactive glass exhibits superior thermal stability compared to quartz glass in laboratory crucibles, maintaining structural integrity at temperatures up to 1350degC, whereas quartz glass typically withstands up to 1200degC before deformation occurs. The unique composition of bioactive glass allows for enhanced resistance to thermal shock and minimal thermal expansion, reducing the risk of cracking during rapid temperature changes. Quartz glass, while chemically inert, is more prone to sudden fracture under thermal cycling due to its higher thermal expansion coefficient and lower strain tolerance.
Chemical Resistance and Reactivity
Bioactive glass exhibits higher chemical reactivity due to its ionic composition, making it less resistant to acidic and basic laboratory environments compared to quartz glass. Quartz glass offers exceptional chemical resistance, maintaining stability against strong acids, bases, and high-temperature reactions, ideal for crucibles used in corrosive or high-purity chemical processes. The superior inertness of quartz glass reduces contamination risks in sensitive experiments, whereas bioactive glass is more prone to surface degradation and ion exchange under aggressive chemical conditions.
Durability and Mechanical Strength
Bioactive glass offers moderate durability and mechanical strength suitable for certain laboratory crucible applications but lacks the robustness of quartz glass, which exhibits exceptional thermal shock resistance and superior mechanical stability at high temperatures. Quartz glass's high purity and crystalline structure enable it to withstand repeated heating cycles without significant deformation or cracking, making it ideal for rigorous laboratory processes. Bioactive glass may degrade under prolonged thermal stress, whereas quartz glass maintains its structural integrity, ensuring long-term reliability in demanding experimental environments.
Applications in Laboratory Settings
Bioactive glass crucibles offer superior chemical resistance and bio-compatibility, making them ideal for applications involving biomaterials synthesis and cell culture experiments in laboratory settings. Quartz glass crucibles excel in high-temperature stability and transparency, which facilitates precise thermal analysis and spectroscopic studies at elevated temperatures. Laboratories frequently select quartz glass for its purity and inertness in chemical reactions, while bioactive glass is preferred when interaction with biological compounds or controlled degradation is required.
Cost and Availability Analysis
Bioactive glass crucibles generally cost more than quartz glass due to the specialized raw materials and manufacturing processes required for their bioactivity properties. Quartz glass crucibles are widely available and benefit from large-scale production, leading to lower prices and easier procurement in laboratory settings. Cost-efficiency and availability make quartz glass the preferred choice for routine high-temperature laboratory applications, while bioactive glass is reserved for niche uses demanding specific biocompatibility or chemical durability.
Safety Considerations
Bioactive glass offers enhanced biocompatibility and lower thermal expansion compared to quartz glass, reducing the risk of cracking under rapid temperature changes in laboratory crucibles. Quartz glass provides superior chemical resistance and higher temperature stability, but it can pose safety hazards due to its brittleness and potential to shatter under thermal shock. Selecting bioactive glass improves user safety in applications involving biological samples, while quartz glass requires careful handling to prevent breakage and contamination.
Choosing the Right Crucible Material
Selecting the appropriate laboratory crucible material involves comparing bioactive glass and quartz glass based on thermal stability and chemical resistance. Bioactive glass offers superior biocompatibility and enhanced bioactivity, making it suitable for specialized biomedical applications, while quartz glass provides exceptional thermal shock resistance and purity ideal for high-temperature reactions. Understanding the specific temperature range, chemical exposure, and experimental requirements ensures optimal crucible performance and longevity.
Infographic: Bioactive glass vs Quartz glass for Laboratory crucible
 azmater.com
azmater.com