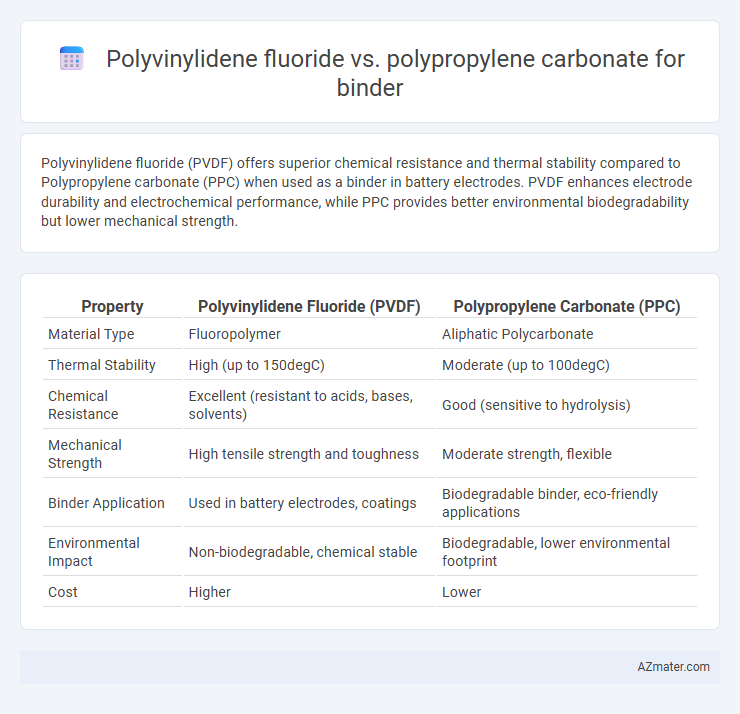
Polyvinylidene fluoride (PVDF) offers superior chemical resistance and thermal stability compared to Polypropylene carbonate (PPC) when used as a binder in battery electrodes. PVDF enhances electrode durability and electrochemical performance, while PPC provides better environmental biodegradability but lower mechanical strength.
Table of Comparison
| Property | Polyvinylidene Fluoride (PVDF) | Polypropylene Carbonate (PPC) |
|---|---|---|
| Material Type | Fluoropolymer | Aliphatic Polycarbonate |
| Thermal Stability | High (up to 150degC) | Moderate (up to 100degC) |
| Chemical Resistance | Excellent (resistant to acids, bases, solvents) | Good (sensitive to hydrolysis) |
| Mechanical Strength | High tensile strength and toughness | Moderate strength, flexible |
| Binder Application | Used in battery electrodes, coatings | Biodegradable binder, eco-friendly applications |
| Environmental Impact | Non-biodegradable, chemical stable | Biodegradable, lower environmental footprint |
| Cost | Higher | Lower |
Introduction to Binder Materials in Energy Storage
Polyvinylidene fluoride (PVDF) and polypropylene carbonate (PPC) are widely used binder materials in energy storage devices, crucial for maintaining electrode integrity and enhancing ionic conductivity. PVDF offers excellent chemical stability and mechanical strength, making it a preferred choice in lithium-ion batteries, whereas PPC provides better biodegradability and higher flexibility but lower chemical resistance. Selecting the appropriate binder affects electrode performance, cycle life, and overall device efficiency in energy storage applications.
Overview of Polyvinylidene Fluoride (PVDF) as a Binder
Polyvinylidene fluoride (PVDF) is widely used as a binder in battery electrodes due to its excellent chemical resistance, strong adhesion properties, and high thermal stability. Its semi-crystalline structure enables effective binding with active materials, facilitating consistent ion transport and mechanical integrity in lithium-ion batteries. Compared to Polypropylene carbonate, PVDF offers superior durability and electrochemical performance, making it a preferred choice in high-performance energy storage applications.
Understanding Polypropylene Carbonate (PPC) as a Binder
Polypropylene carbonate (PPC) is a biodegradable polymer known for its excellent film-forming properties, making it an effective binder in various applications such as coatings and composites. Compared to polyvinylidene fluoride (PVDF), PPC offers superior environmental benefits due to its compostability and reduced ecological impact. PPC's lower melting point and good chemical resistance enhance its processability and adhesion performance, providing a sustainable alternative in binder formulations.
Chemical Structure Comparison: PVDF vs PPC
Polyvinylidene fluoride (PVDF) features a highly polar fluorinated backbone with repeating -(CH2-CF2)- units, providing excellent chemical resistance and thermal stability, which enhances its performance as a binder in harsh environments. Polypropylene carbonate (PPC) possesses a carbonate linkage in its backbone with repeating -(C3H6O2)- units, offering moderate polarity and biodegradability but lower chemical and thermal resistance compared to PVDF. The distinct difference in polarity and backbone stability between PVDF's fluorinated chains and PPC's carbonate groups significantly influences their binder properties in applications requiring durability and environmental compatibility.
Mechanical and Adhesive Properties
Polyvinylidene fluoride (PVDF) exhibits superior mechanical strength and chemical resistance, making it ideal for binders requiring durability and long-term stability, while polypropylene carbonate (PPC) offers enhanced flexibility and biodegradability with moderate tensile strength. PVDF binders provide excellent adhesive properties on metal and ceramic substrates due to strong polarity and crystallinity, whereas PPC's adhesive performance excels on polymeric and organic surfaces because of its carbonate groups facilitating intermolecular bonding. The choice between PVDF and PPC binders depends on application-specific mechanical demands and substrate compatibility, balancing performance and environmental considerations.
Electrochemical Stability and Compatibility
Polyvinylidene fluoride (PVDF) exhibits superior electrochemical stability with a wide electrochemical window up to 5.5 V versus Li/Li+, making it highly compatible with various electrode materials in lithium-ion batteries. Polypropylene carbonate (PPC), while less electrochemically stable with a narrower oxidation window around 4.0 V, offers better compatibility with flexible electrodes due to its elasticity. PVDF's chemical resistance to electrolytes and solvents enhances binder durability, whereas PPC's biodegradable nature and moderate solubility affect long-term battery performance.
Environmental Impact and Sustainability
Polyvinylidene fluoride (PVDF) exhibits excellent chemical resistance and mechanical strength but poses environmental concerns due to its fluorinated structure, which complicates recycling and can release toxic substances during degradation. Polypropylene carbonate (PPC), derived from carbon dioxide and propylene oxide, offers a more sustainable binder option with enhanced biodegradability and lower carbon footprint, supporting circular economy initiatives. PPC's ability to reduce greenhouse gas emissions during production and its compatibility with composting processes make it increasingly favored in eco-friendly applications over PVDF.
Cost and Scalability Considerations
Polyvinylidene fluoride (PVDF) offers higher chemical resistance and thermal stability compared to polypropylene carbonate (PPC), making it suitable for demanding binder applications despite its higher cost. PPC provides a cost-effective and biodegradable alternative, with easier processability that favors large-scale production but may compromise long-term durability. Scalability of PVDF is often limited by its complex synthesis and higher raw material costs, whereas PPC benefits from renewable feedstocks and simpler polymerization techniques, enhancing its commercial viability.
Performance in Lithium-Ion Battery Applications
Polyvinylidene fluoride (PVDF) exhibits superior chemical stability and excellent adhesion properties, making it the preferred binder for lithium-ion batteries with high capacity electrodes. Polypropylene carbonate (PPC) offers enhanced flexibility and environmental friendliness but generally provides lower electrochemical stability and ionic conductivity compared to PVDF. Performance-wise, PVDF enables better cycle life and capacity retention, while PPC remains promising for sustainable and low-toxicity battery applications.
Future Trends and Research Directions
Polyvinylidene fluoride (PVDF) and Polypropylene carbonate (PPC) are gaining attention as binders due to their distinct chemical stability and biodegradability, respectively. Future trends focus on enhancing PVDF's electrochemical performance through nanocomposite formulations and improving PPC's thermal resistance for broader industrial applications. Research directions emphasize developing hybrid binders that combine PVDF's robustness with PPC's eco-friendly properties to meet evolving sustainability standards in energy storage technologies.
Infographic: Polyvinylidene fluoride vs Polypropylene carbonate for Binder
 azmater.com
azmater.com