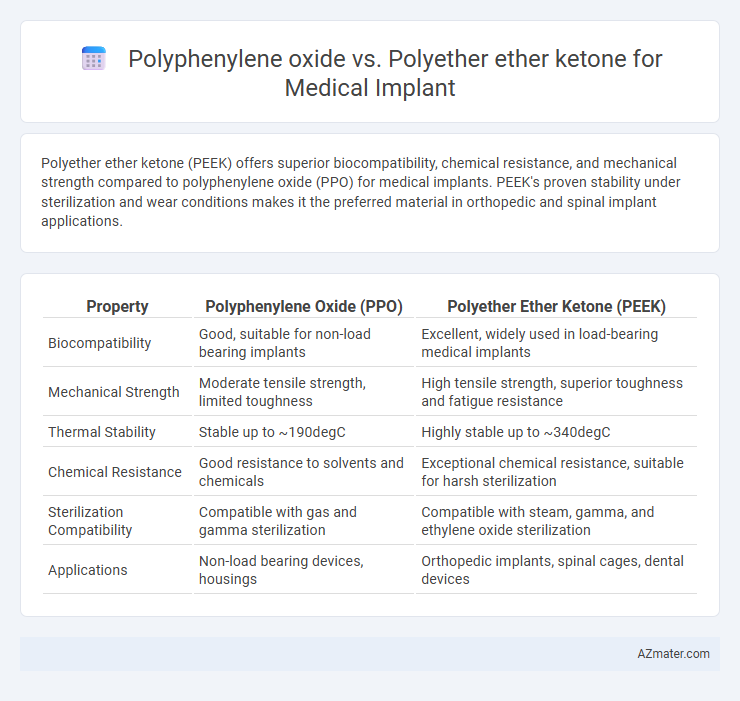
Polyether ether ketone (PEEK) offers superior biocompatibility, chemical resistance, and mechanical strength compared to polyphenylene oxide (PPO) for medical implants. PEEK's proven stability under sterilization and wear conditions makes it the preferred material in orthopedic and spinal implant applications.
Table of Comparison
| Property | Polyphenylene Oxide (PPO) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Biocompatibility | Good, suitable for non-load bearing implants | Excellent, widely used in load-bearing medical implants |
| Mechanical Strength | Moderate tensile strength, limited toughness | High tensile strength, superior toughness and fatigue resistance |
| Thermal Stability | Stable up to ~190degC | Highly stable up to ~340degC |
| Chemical Resistance | Good resistance to solvents and chemicals | Exceptional chemical resistance, suitable for harsh sterilization |
| Sterilization Compatibility | Compatible with gas and gamma sterilization | Compatible with steam, gamma, and ethylene oxide sterilization |
| Applications | Non-load bearing devices, housings | Orthopedic implants, spinal cages, dental devices |
Introduction to High-Performance Polymers in Medical Implants
Polyphenylene oxide (PPO) and polyether ether ketone (PEEK) are high-performance polymers widely utilized in medical implants due to their exceptional mechanical strength, chemical resistance, and biocompatibility. PEEK offers superior wear resistance and thermal stability, making it ideal for load-bearing orthopedic implants, while PPO is valued for its excellent dimensional stability and electrical insulating properties in electronic medical devices. Both polymers contribute to innovation in medical implant technology by enhancing longevity and patient safety through their advanced material characteristics.
Overview of Polyphenylene Oxide (PPO): Structure and Properties
Polyphenylene oxide (PPO) is a high-performance thermoplastic characterized by a rigid aromatic backbone, providing excellent dimensional stability and heat resistance ideal for medical implants. Its inherent biocompatibility, chemical resistance, and low moisture absorption support long-term implant performance and patient safety. PPO offers superior strength-to-weight ratio and electrical insulating properties, making it a preferred choice in applications where durability and reliability are critical.
Overview of Polyether Ether Ketone (PEEK): Structure and Properties
Polyether ether ketone (PEEK) is a semicrystalline thermoplastic polymer characterized by a backbone of alternating ketone and ether groups, offering exceptional thermal stability and mechanical strength. Its high biocompatibility, chemical resistance, and fatigue durability make it an ideal material for medical implants, including spinal cages and dental devices. PEEK's modulus closely matches that of human bone, reducing stress shielding and enhancing implant longevity compared to polymers like polyphenylene oxide.
Mechanical Strength: PPO vs PEEK in Medical Applications
Polyether ether ketone (PEEK) exhibits superior mechanical strength compared to polyphenylene oxide (PPO) in medical implant applications, delivering exceptional tensile strength around 90-100 MPa and high impact resistance, critical for load-bearing implants. PPO offers moderate mechanical properties with lower tensile strength near 55-70 MPa, making it suitable for non-load-bearing or temporary implant components. The enhanced fatigue resistance and dimensional stability of PEEK make it preferable for demanding orthopedic and spinal implant devices where long-term durability is essential.
Biocompatibility and Bioinertness: Essential Considerations
Polyether ether ketone (PEEK) demonstrates superior biocompatibility and bioinertness compared to polyphenylene oxide (PPO), making it highly suitable for medical implants that require long-term stability and minimal tissue reaction. PEEK's molecular structure provides excellent chemical resistance and low immunogenicity, reducing the risk of inflammatory responses and implant failure. PPO, while durable and thermally stable, lacks the extensive clinical validation and bioinert profile that PEEK offers for critical implant applications.
Sterilization Resistance and Long-Term Stability
Polyether ether ketone (PEEK) exhibits superior sterilization resistance and long-term stability compared to polyphenylene oxide (PPO) in medical implant applications due to its high thermal and chemical resistance. PEEK maintains mechanical integrity and biocompatibility after repeated steam, gamma, and ethylene oxide sterilization cycles, making it ideal for implants requiring rigorous sterilization protocols. PPO, while usable in some medical devices, shows lower resistance to high-temperature sterilization and potential degradation over extended periods, limiting its effectiveness for long-term implant durability.
Processing, Machinability, and Manufacturing Constraints
Polyphenylene oxide (PPO) offers excellent dimensional stability and ease of processing with conventional thermoplastic methods, making it suitable for intricate medical implant designs, while Polyether ether ketone (PEEK) demands higher processing temperatures and specialized equipment due to its superior thermal resistance and chemical stability. In terms of machinability, PPO is easier to machine with lower tool wear and faster cycle times, whereas PEEK requires slower machining speeds and high-performance tooling to maintain precision without degrading material properties. Manufacturing constraints for PPO involve limitations in sterilization methods and long-term mechanical strength under physiological conditions, while PEEK's biocompatibility and resistance to radiation sterilization expand its application scope despite higher production costs and complex thermal processing protocols.
Regulatory Approvals and Medical Standards Compliance
Polyether ether ketone (PEEK) demonstrates superior regulatory approvals and compliance with rigorous medical standards, including FDA 510(k) clearance and ISO 13485 certification, making it a preferred choice for long-term medical implants due to its biocompatibility and sterilization resilience. Polyphenylene oxide (PPO), while biocompatible and stable, lacks the extensive clinical approval history and specific certifications that PEEK holds, limiting its widespread adoption in critical implantable devices. The robust regulatory framework supporting PEEK ensures enhanced patient safety and device reliability, essential for successful medical implant applications.
Cost-Effectiveness and Supply Chain Factors
Polyphenylene oxide (PPO) offers a more cost-effective solution for medical implants due to its lower raw material costs and simpler manufacturing processes compared to Polyether ether ketone (PEEK). PPO benefits from a broader and more stable supply chain, ensuring consistent availability and reducing lead times critical for medical device production. In contrast, PEEK's higher material costs and supply chain complexities can increase overall expenses, impacting budget-sensitive healthcare applications.
Future Perspectives: Innovations and Trends in Medical Implant Polymers
Polyphenylene oxide (PPO) and polyether ether ketone (PEEK) demonstrate promising advancements in medical implant polymers, with PEEK offering superior mechanical strength, chemical resistance, and biocompatibility tailored for load-bearing applications. Innovations focus on enhancing PPO's antimicrobial and bioactive surface modifications, potentially expanding its usage in non-load-bearing implants and drug delivery systems. Trends emphasize integrating nanotechnology and biofunctionalization to improve osseointegration and long-term implant performance, positioning PEEK and PPO at the forefront of next-generation medical implant materials.
Infographic: Polyphenylene oxide vs Polyether ether ketone for Medical Implant
 azmater.com
azmater.com