Polylactic acid (PLA) offers biodegradability and biocompatibility ideal for temporary medical implants, while Polyetheretherketone (PEEK) provides superior mechanical strength, chemical resistance, and long-term stability for permanent implant applications. Medical devices requiring load-bearing and durability benefits commonly select PEEK, whereas PLA suits resorbable scaffolds and drug delivery systems.
Table of Comparison
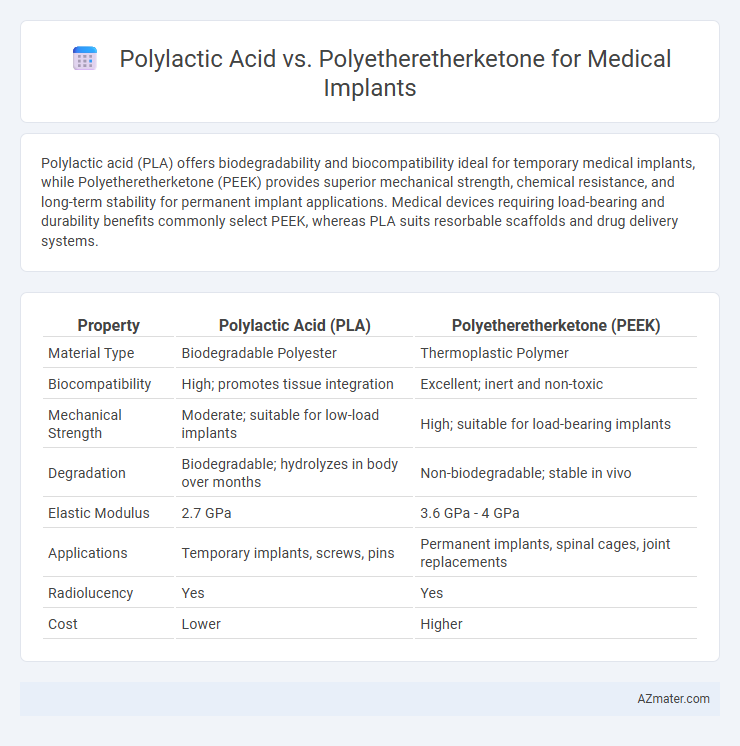
| Property | Polylactic Acid (PLA) | Polyetheretherketone (PEEK) |
|---|---|---|
| Material Type | Biodegradable Polyester | Thermoplastic Polymer |
| Biocompatibility | High; promotes tissue integration | Excellent; inert and non-toxic |
| Mechanical Strength | Moderate; suitable for low-load implants | High; suitable for load-bearing implants |
| Degradation | Biodegradable; hydrolyzes in body over months | Non-biodegradable; stable in vivo |
| Elastic Modulus | 2.7 GPa | 3.6 GPa - 4 GPa |
| Applications | Temporary implants, screws, pins | Permanent implants, spinal cages, joint replacements |
| Radiolucency | Yes | Yes |
| Cost | Lower | Higher |
Introduction to Polylactic Acid (PLA) and Polyetheretherketone (PEEK)
Polylactic acid (PLA) is a biodegradable thermoplastic derived from renewable resources such as corn starch, known for its excellent biocompatibility and suitability in resorbable medical implants. Polyetheretherketone (PEEK) is a high-performance engineering thermoplastic distinguished by its exceptional mechanical strength, chemical resistance, and stability under sterilization, making it a preferred choice for durable, long-term implants. Both materials offer distinct advantages in medical applications, with PLA favored for temporary, bioresorbable devices and PEEK used in load-bearing, permanent implant solutions.
Chemical Structure Comparison: PLA vs PEEK
Polylactic acid (PLA) is a biodegradable aliphatic polyester derived from renewable resources, characterized by its ester linkages that promote hydrolytic degradation, whereas polyetheretherketone (PEEK) is a semi-crystalline thermoplastic polymer featuring aromatic ketone and ether groups that confer exceptional chemical resistance and thermal stability. The chemical backbone of PLA consists of repeating lactic acid units with ester functional groups, making it susceptible to enzymatic and hydrolytic breakdown, ideal for temporary medical implants. In contrast, PEEK's rigid aromatic rings and ketone linkages create a high-performance polymer structure offering long-term biostability and mechanical strength, suitable for permanent implant applications.
Biocompatibility of PLA and PEEK in Medical Implants
Polylactic acid (PLA) exhibits excellent biocompatibility due to its biodegradability and non-toxic degradation products, making it suitable for temporary medical implants that gradually dissolve in the body. Polyetheretherketone (PEEK) offers superior biocompatibility with high chemical resistance, mechanical strength, and a stable inert nature, ideal for permanent implants requiring long-term durability. Both materials facilitate bone integration, but PLA's resorbable properties contrast with PEEK's non-degradable, high-performance characteristics essential for load-bearing implant applications.
Mechanical Properties: Strength, Flexibility, and Durability
Polylactic acid (PLA) exhibits moderate strength and flexibility suitable for biodegradable medical implants but lacks the long-term durability required for load-bearing applications. Polyetheretherketone (PEEK) shows superior mechanical properties with high tensile strength, excellent flexibility, and exceptional durability, making it ideal for permanent implants exposed to mechanical stress. The choice between PLA and PEEK depends on implant duration and mechanical demands, where PEEK outperforms PLA in strength retention and fatigue resistance.
Degradation and Bioactivity in Biological Environments
Polylactic acid (PLA) exhibits a predictable hydrolytic degradation profile, breaking down into lactic acid that is metabolized by the body, making it suitable for resorbable medical implants. Polyetheretherketone (PEEK) demonstrates exceptional chemical stability and resists biodegradation, maintaining structural integrity over extended periods in biological environments. PLA offers higher bioactivity with potential for cellular interaction and tissue integration, whereas PEEK's bioinert nature often requires surface modification to enhance osseointegration in implant applications.
Imaging Compatibility: MRI and CT Visibility
Polylactic acid (PLA) demonstrates limited MRI artifact formation due to its non-metallic, amorphous structure, ensuring clearer imaging during follow-up evaluations compared to Polyetheretherketone (PEEK). PEEK exhibits minimal CT visibility because of its radiolucency, which can complicate the precise localization of implants in computed tomography scans. The radiographic compatibility of PLA allows enhanced MRI and CT imaging, facilitating improved diagnostic accuracy in post-implant assessments.
Manufacturing Processes and Design Flexibility
Polylactic acid (PLA) offers advantages in manufacturing processes, including ease of 3D printing and injection molding, enabling rapid prototyping and customizable implant designs with biodegradability. Polyetheretherketone (PEEK) requires high-temperature processing such as compression molding or extrusion, offering superior mechanical strength and chemical resistance, which supports durable, load-bearing medical implants. PEEK's design flexibility is enhanced by machinability and compatibility with advanced techniques like CNC milling, facilitating complex geometries for long-term orthopedic and dental applications.
Clinical Applications and Case Studies
Polylactic acid (PLA) and Polyetheretherketone (PEEK) are widely studied biomaterials for medical implants, with PLA commonly used in biodegradable applications such as bone fixation devices and tissue engineering scaffolds due to its excellent biocompatibility and controlled degradation rate. PEEK is favored in load-bearing orthopedic implants and spinal surgeries, offering superior mechanical strength, chemical resistance, and radiolucency, as demonstrated in numerous clinical case studies showcasing its long-term stability and reduced inflammatory response. Comparative analyses reveal that while PLA suits temporary implants requiring bioresorption, PEEK excels in permanent implant applications demanding durability and high performance under physiological conditions.
Regulatory Approval and Safety Considerations
Polylactic acid (PLA) is widely recognized for its biodegradability and has obtained extensive regulatory approvals such as FDA clearance for resorbable medical implants, offering predictable degradation profiles that enhance patient safety. Polyetheretherketone (PEEK) is a high-performance thermoplastic known for its mechanical strength and biocompatibility, with FDA and CE approvals for long-term implantable devices due to its chemical stability and minimal inflammatory response. Safety considerations emphasize PLA's suitability for temporary applications with metabolic resorption, whereas PEEK is preferred for permanent implants requiring durability and resistance to sterilization processes.
Future Trends and Innovations in PLA and PEEK Implants
Polylactic acid (PLA) and polyetheretherketone (PEEK) are pivotal materials in medical implants, with PLA advancing through biodegradable properties and enhanced biocompatibility via nanocomposite integration. Innovations in PEEK focus on surface modifications and bioactive coatings to improve osseointegration and mechanical performance in load-bearing implants. Future trends emphasize smart implant developments utilizing PLA's resorbable nature and PEEK's mechanical strength combined with sensor integration for real-time monitoring and personalized medicine.
Infographic: Polylactic acid vs Polyetheretherketone for Medical Implant
 azmater.com
azmater.com