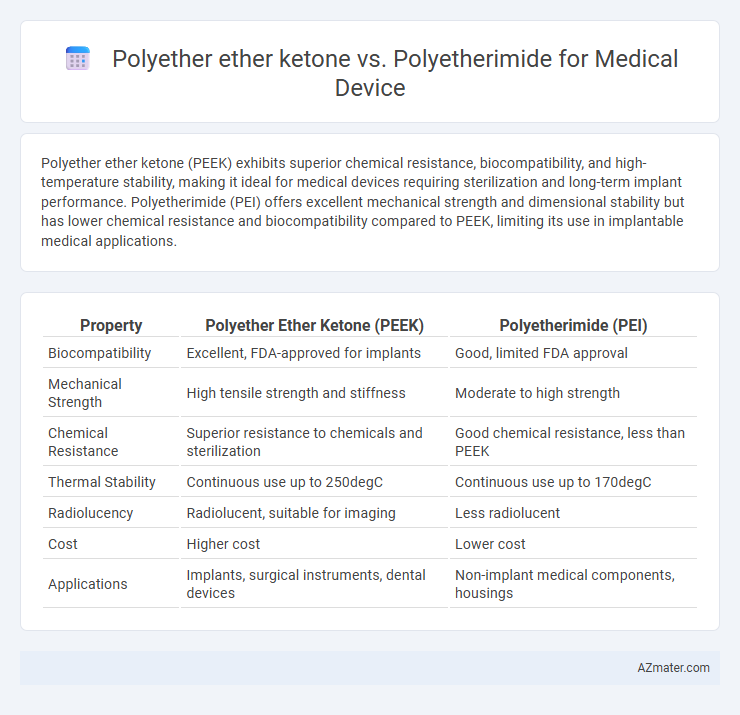
Polyether ether ketone (PEEK) exhibits superior chemical resistance, biocompatibility, and high-temperature stability, making it ideal for medical devices requiring sterilization and long-term implant performance. Polyetherimide (PEI) offers excellent mechanical strength and dimensional stability but has lower chemical resistance and biocompatibility compared to PEEK, limiting its use in implantable medical applications.
Table of Comparison
| Property | Polyether Ether Ketone (PEEK) | Polyetherimide (PEI) |
|---|---|---|
| Biocompatibility | Excellent, FDA-approved for implants | Good, limited FDA approval |
| Mechanical Strength | High tensile strength and stiffness | Moderate to high strength |
| Chemical Resistance | Superior resistance to chemicals and sterilization | Good chemical resistance, less than PEEK |
| Thermal Stability | Continuous use up to 250degC | Continuous use up to 170degC |
| Radiolucency | Radiolucent, suitable for imaging | Less radiolucent |
| Cost | Higher cost | Lower cost |
| Applications | Implants, surgical instruments, dental devices | Non-implant medical components, housings |
Introduction to Polyether Ether Ketone (PEEK) and Polyetherimide (PEI)
Polyether ether ketone (PEEK) is a high-performance thermoplastic known for its excellent mechanical strength, chemical resistance, and biocompatibility, making it ideal for medical device applications such as implants and surgical instruments. Polyetherimide (PEI) offers high thermal stability, good dimensional stability, and inherent flame resistance, suitable for complex medical components requiring sterilization and structural durability. Both PEEK and PEI provide unique advantages in medical devices, with PEEK excelling in load-bearing applications and PEI favored for precise, heat-resistant parts.
Chemical Structure Comparison
Polyether ether ketone (PEEK) and polyetherimide (PEI) are high-performance polymers used in medical devices, distinguished by their chemical structures. PEEK features a repeating unit with ether and ketone linkages, offering exceptional chemical resistance and mechanical strength, while PEI contains ether and imide groups, providing excellent thermal stability and dimensional stability. The ketone groups in PEEK contribute to its superior wear resistance, whereas the imide rings in PEI enhance rigidity and resistance to hydrolysis, affecting their suitability for various sterilization methods and implantable applications.
Mechanical Properties: Strength and Durability
Polyether ether ketone (PEEK) exhibits exceptional mechanical strength and durability, with a tensile strength typically around 90-100 MPa and excellent resistance to fatigue, making it ideal for long-term medical device implants. Polyetherimide (PEI) offers good mechanical properties, including tensile strength near 110 MPa, but generally falls short in fatigue resistance and long-term durability compared to PEEK in demanding medical applications. The superior chemical resistance and high-temperature stability of PEEK contribute to its enhanced mechanical reliability and performance in sterilizable, high-stress environments.
Biocompatibility and Safety Profiles
Polyether ether ketone (PEEK) exhibits superior biocompatibility with excellent resistance to sterilization methods, making it highly suitable for long-term medical implants and devices. Polyetherimide (PEI) offers good mechanical strength but shows lower biocompatibility and potential cytotoxicity concerns compared to PEEK, limiting its use in direct tissue-contact applications. Safety profiles favor PEEK due to its inert nature, minimizing inflammatory responses and wear debris risks critical for patient safety in medical device manufacturing.
Sterilization Methods Compatibility
Polyether ether ketone (PEEK) demonstrates superior compatibility with multiple sterilization methods including autoclaving, gamma radiation, and ethylene oxide, maintaining mechanical integrity and biocompatibility essential for medical devices. Polyetherimide (PEI), while resistant to ethylene oxide and gamma radiation, exhibits reduced stability under repeated autoclave cycles, limiting its use in steam sterilization processes. Selecting PEEK for medical devices ensures consistent performance across diverse sterilization protocols critical for high-quality healthcare applications.
Thermal and Chemical Resistance
Polyether ether ketone (PEEK) exhibits exceptional thermal resistance with a melting point around 343degC, maintaining structural integrity under prolonged high-temperature conditions, making it highly suitable for medical devices requiring sterilization via autoclaving. Polyetherimide (PEI) offers superior chemical resistance against a broad range of solvents and acids but has a lower maximum continuous use temperature of approximately 170degC, limiting its application where high-temperature exposure is critical. The choice between PEEK and PEI for medical device components depends on the balance between thermal endurance and chemical resistance needs, with PEEK favored for extreme thermal environments and PEI for chemical exposure without high heat demands.
Applications in Implantable and Non-Implantable Medical Devices
Polyether ether ketone (PEEK) excels in implantable medical device applications due to its outstanding biocompatibility, high mechanical strength, and resistance to sterilization processes, making it ideal for spinal implants, orthopedic devices, and dental implants. Polyetherimide (PEI) is commonly used in non-implantable medical devices such as surgical instruments, diagnostic equipment components, and sterilization trays because of its excellent thermal stability, chemical resistance, and dimensional stability. Both materials meet strict regulatory standards, but PEEK's superior bioinertness and fatigue resistance make it preferable for long-term implants, while PEI's high-performance thermoplastic properties suit extensive non-implantable device applications.
Manufacturing and Processing Considerations
Polyether ether ketone (PEEK) offers superior chemical resistance and high-temperature stability, making it ideal for sterilization processes in medical device manufacturing, while polyetherimide (PEI) provides easier moldability and faster cycle times due to its lower melting point. PEEK requires higher processing temperatures around 370degC and more precise thermal control, demanding advanced injection molding equipment compared to PEI's processing temperature near 340degC. Both materials ensure biocompatibility, but manufacturing efficiency and equipment costs often guide the selection between PEEK's robustness and PEI's processing advantages for medical applications.
Regulatory Approvals and Compliance
Polyether ether ketone (PEEK) is extensively recognized for its biocompatibility and FDA compliance, widely approved for implants and surgical instruments in medical devices. Polyetherimide (PEI), while strong and heat-resistant, has more limited FDA clearance, often requiring additional biocompatibility testing for long-term implant use. Regulatory approvals for PEEK emphasize its chemical stability and mechanical durability under sterilization processes, making it a preferred choice in highly regulated medical applications.
Cost Analysis and Market Trends
Polyether ether ketone (PEEK) typically incurs higher material and processing costs than polyetherimide (PEI), influencing overall medical device manufacturing budgets due to PEEK's superior chemical resistance and biocompatibility. Market trends indicate a growing preference for PEEK in implantable devices because of its mechanical strength and sterilization resilience, despite PEI's cost advantage in non-implantable applications. Recent industry reports forecast increased demand for both polymers, with PEI expanding in diagnostic equipment while PEEK dominates in high-performance surgical tools and orthopedic implants.
Infographic: Polyether ether ketone vs Polyetherimide for Medical Device
 azmater.com
azmater.com